First detailed picture of a cancer-related cell enzyme in action on a chromosome unit
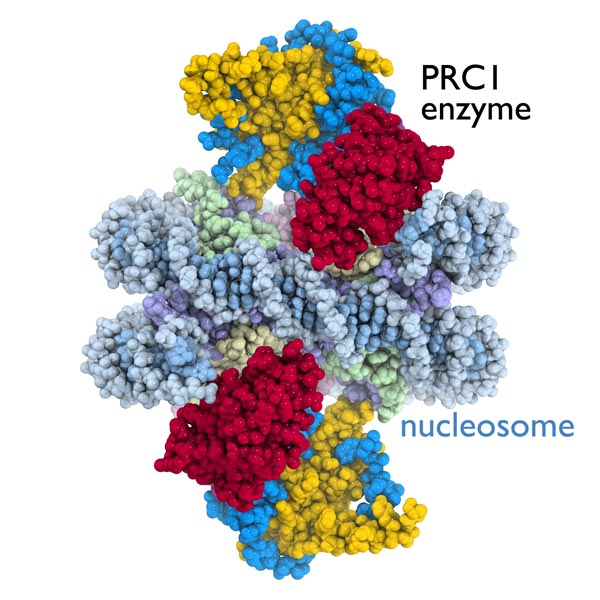
This image is the first detailed picture of the crystal structure of a gene-regulation enzyme while it is working on a nucleosome -- a fundamental component of the chromosomes that provide structure and organization for an organism's genes. Nucleosomes are key targets of the enzymes that conduct genetic processes critical for life. This image reveals the crystal structure of the PRC1 enzyme (yellow, blue and red) bound to the nucleosome (DNA in light blue, histone proteins in purple, light green, light yellow and pink) This image was obtained in the lab of Song Tan at Penn State University and was published in the print edition of the journal Nature on October 30, 2014. Credit: Song Tan lab, Penn State University
Enzymes like PRC1 turn on or turn off the activity of genes in a cell by manipulating individual chromosome units called nucleosomes. “The nucleosome is a key target of the enzymes that conduct genetic processes critical for life,” said Song Tan, professor of biochemistry and molecular biology at Penn State University and the leader of the study's research team.
The Penn State scientists obtained the first crystal structure of a gene regulation enzyme while it is working on a nucleosome. The image reveals previously unknown information about how the enzyme attaches to its nucleosome target.
Before this study, scientists had been unable to picture exactly how cancer-related enzymes in the PRC1 group interacted with a nucleosome to control gene activity. The study is also the first to determine the crystal structure of a multisubunit protein complex bound to a nucleosome, which itself is a complex assembly of DNA and 4 histone proteins.
The research is the culmination of over 12 years of research by the Tan laboratory to capture an image of this important class of enzymes bound to the nucleosome. His lab earlier had determined the first structure of another nucleosome-bound protein, RCC1.
“This is the second important structure from the Tan lab to date of a nucleosome in complex with a protein known to interact with and modify chromatin behavior, which in turn can influence human gene expression,” said Peter Preusch, Ph.D., of the National Institutes of Health's National Institute of General Medical Sciences, which partially funded the research. “Along with Dr. Tan's previous work detailing a nucleosome bound to the key regulatory protein, RCC1, this new structure adds to our knowledge of how proteins can regulate the structure and function of our genetic material.”
The research project was proposed and executed by team member Robert K. McGinty, a Damon Runyon postdoctoral fellow at Penn State. McGinty and Ryan C. Henrici, an undergraduate in the Penn State Schreyer Honors College, grew crystals of the PRC1 enzyme bound to the nucleosome.
The team then solved the three-dimensional structure of this large molecular assembly by X-ray crystallography. “We are excited about this crystal structure because it provides new paradigms for understanding how chromatin enzymes function,” McGinty said.
The study performed in the Penn State Center for Eukaryotic Gene Regulation provides unexpected insight into the workings of the BRCA1 breast-cancer-associated tumor-suppressor protein. Like PRC1, BRCA1 is a chromatin enzyme that shares a similar activity on the nucleosome. Tan said, “Our study suggests that BRCA1 and PRC1 employ a similar mechanism to anchor to the nucleosome”. Tan and his team now are working to visualize how BRCA1 and other disease-related chromatin enzymes interact with the nucleosome.
This research was supported by grants from the National Institutes of Health, the Damon Runyon Cancer Research Foundation and Penn State University.
Contacts
Song Tan: sxt30@psu.edu, 814-865-3355
Barbara Kennedy (PIO): science@psu.edu, 814-863-4682
Media Contact
More Information:
http://science.psu.edu/news-and-events/2014-news/Tan10-2014-2All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















