Diamond Wires
<br>
Carbon-based nanomaterials have unique properties that make them useful for many technical applications, including lightweight construction, electronics, energy generation, environmental technology, and medicine.
In the journal Angewandte Chemie, an international team of researchers has now introduced a new process for the production of especially fine carbon nanowires from carbon in the diamond configuration. In this process, molecules with a diamond-like structure are linked together inside a carbon nanotube.
On the occasion of the 125th anniversary of Angewandte Chemie, a one-day symposium is held on March 12 with several Nobel laureates. Learn more and join the webcast at chemistryviews.org/angewandtechemie125.
Carbon occurs in several configurations. Graphite and diamond have long been known. While graphite consists of two-dimensional, honeycomb-like sheets of carbon, diamonds are three- dimensional, cage-like structures consisting of puckered six-carbon rings. A variety of new nanoconfigurations have also been discovered: fullerenes, carbon nanotubes, graphene (graphite monolayers), nanodiamonds, and diamondoids. Diamondoids are actually cycloalkane molecules with a skeleton of carbon configured in “cages”, like diamond. They can be viewed as miniature diamonds with hydrogen atoms bound to their outer surfaces.
Nanowires are needed for many nanoscale applications. Various types of nanowire have been produced, including some with diameters ranging from about 50 to 100 nm, made of carbon in the diamond configuration. A team of researchers from Japan, China, Germany, and the USA wanted to reduce the dimensions of nanowires further into the sub-nanometer range. Such tiny wires could be of use in the tips of scanning tunneling microscopes, which are devices that can be used to scan the topology of a surface to produce extremely high-resolution images.
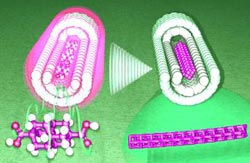
Researchers led by Hisanori Shinohara at Nagoya University (Japan) came up with the idea of fusing diamondoids into longer, superfine wires.
To make this work they had to resort to a trick: carbon nanotube “molds”. For their starting material, the scientists chose diadamantane, a diamondoid made of two diamond-like cages. They attached a carbonic acid group at each end of these molecules. The molecules are transferred to the gas phase for the synthetic procedure. They are sucked into the tiny carbon nanotubes by capillary action. The best nanotube molds were found to be those with an inner diameter of about 1.3 nm.
Within the nanotubes, the diamondoids line up like a string of pearls. Heating these to about 600 °C under a hydrogen atomsphere causes a polymerization/fusion reaction in which the individual diamondoid molecules link up through their carbonic acid groups to form a long “wire” with a diameter of about 0.78 nm. The cage-like structure is maintained.
By using theoretical calculations and various analytical techniques, the scientists were able to demonstrate that the carbon in the wires is indeed in a diamond-like structure.
Currently, the scientists are elaborating an ultrasonication extraction technique for releasing the nanowires from the surrounding carbon nanotubes.
About the Author
Dr Hisanori Shinohara is a Professor at Nagoya University with appointments in Physical Chemistry and Materials Science. His main specialty is nanoscience and nanotechnology on carbon nanomaterials such as fullerenes, carbon nanotubes and graphene. He is also the Dean of the Graduate School of Science.
Author: Hisanori Shinohara, Nagoya University (Japan),
http://www3.chem.nagoya-u.ac.jp/wordpress/?page_id=1101&lang=en
Title: Evidence of Diamond Nanowires Formed inside Carbon Nanotubes from Diamantane Dicarboxylic Acid
Angewandte Chemie International Edition, Permalink to the article: http://dx.doi.org/10.1002/anie.201209192
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















