Determining the atomic structure of natural products more rapidly and accurately
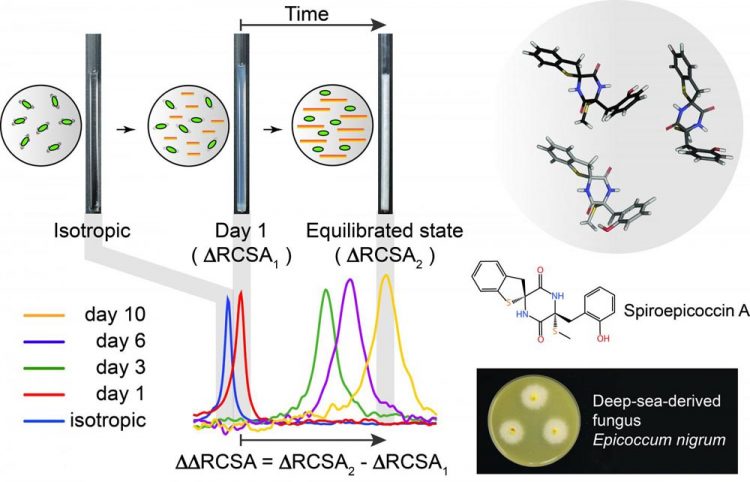
Measuring the residual chemical shift anisotropy in a liquid crystalline medium. The method was used to determine the stereochemistry of spiroepicoccin A, a recently discovered natural product. The novel natural product was isolated from the deep-sea fungus Epicoccum nigrum, which can be found at depths of more than 4,500 meters. Credit: Songhwan Hwang, FMP
A new NMR-based method, developed at the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP), now simplifies the analysis and produces more accurate results. The work has been published in the Journal of the American Chemical Society.
Natural products are present in antibiotics, painkillers and cancer drugs, playing a key role in around 60 percent of all FDA approved drugs. Plants, fungi and sessile marine organisms are particularly promising sources, because many of them possess chemical defenses to deter predators. However, identification of potential drug candidates is a challenge.
First, researchers must accurately determine the structure and stereochemistry (the spatial arrangement of atoms) of the molecules. Without this information, chemists will be unable to synthesize the molecules and develop them into drugs. Moreover, the structure is needed to establish whether the molecule has previously been discovered.
Besides the X-ray diffraction method, which can only be applied to the crystallizable molecules, chemists usually use nuclear magnetic resonance (NMR) spectroscopy for structure determination. Most recently, the NMR-based parameter “residual chemical shift anisotropy” has taken on particular importance in this context.
Studies from the past two to three years have shown that this parameter facilitates the very accurate determination of the structure and stereochemistry of organic molecules. However, this requires the use of special instruments that are not available in all laboratories. And then there is the matter of the time-consuming methods of analysis involved in data analysis.
Simplified method produces more accurate results
Researchers from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) have now developed a method that enables the residual chemical shift anisotropy to be measured much more easily and effectively.
Partners from China (Institute of Oceanology, Chinese Academy of Sciences and South Central University for Nationalities) and Brazil (Universidade Federal de Pernambuco) were also involved in the work, which has now been published in the Journal of the American Chemical Society.
“The NMR-based method we have developed enables chemists to determine the stereochemistry of novel natural products with greater accuracy and efficiency,” explained Dr. Han Sun from FMP, who led the study. ” Furthermore, the method is very easy to use, making it accessible to all chemists.”
The experiment involves bringing together natural products with a commercially available peptide with a sequence of AAKLVFF. Dissolved in methanol, the peptides are transformed into liquid crystals, giving the natural products a weak orientation in the magnetic field.
“This particular orientation enables us to measure the residual chemical shift anisotropy of the molecules as a parameter, which in turn provides accurate information about their structure and stereochemistry,” stated the chemist Sun, describing the new method.
The example of thalidomide shows how important it is to determine the stereochemistry of compounds correctly. Besides having a sedative effect (hypnotic), the compound thalidomide also has an adverse developmental effect, which is attributable to its two mirror-image forms (R)-thalidomide and (S)-thalidomide.
Analysis of exotic natural products from the ocean
For their current study, the researchers used a previously unexplored natural product: spiroepicoccin A was isolated from marine microorganisms by the Chinese partners. The substance, obtained from a depth of more than 4,500 meters, only has a few hydrogen atoms attached to its stereocenters, posing a challenge to established NMR methods. Thanks to the new measurement method, however, the structure and stereochemistry of the natural product were unambiguously elucidated.
“Even though our method enables us to measure only the relative and not the absolute stereochemistry as yet, our work makes an important contribution to simplifying the determination of challenging natural products,” remarked Sun. It appears that pharmaceutical companies have already expressed an interest “because the method accelerates the development of new drugs, which is also our aim.”
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















