Defense against viruses or autoimmune disorder? When the phosphate decides …
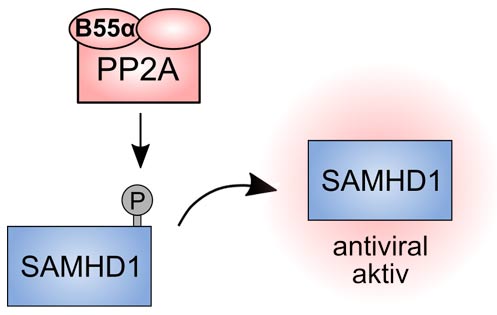
Schematic representation of the dephosphorylation of the restriction factor SAMHD1. Source: PEI
Restriction factors inhibit viral infection and proliferation (replication) in body cells. SAMHD1 (sterile alpha motif and histidine-aspartate (HD)-domain-containing protein 1) is such a restriction factor. It was identified as an important protein acting antivirally against HIV-1 (human immunodeficiency virus 1), but it was also found to have other functions.
Thus, it was shown that mutations in the SAMHD1 gene go hand in hand with the loss of its function and may that way cause cancer and autoimmune disorders. Accordingly, there is a major interest in understanding the mode of action and regulation of this molecule better.
SAMHD1 regulates the amount of important building blocks for the formation of cellular DNA available in cells, the dNTPs (desoxynucleotide triphosphates). By attaching a phosphate group (phosphorylation) to the amino acid at positon T592, SAMHD1 becomes able to influence DNA sections that “stagnate“ during DNA duplication in such a way that the DNA replication (duplication) can be continued, thus preventing chronic inflammation. In a non-phosphorylated state, on the other hand, SAMHD1 has an antiviral effect.
Dr. Renate König, head of the research group “Cellular aspects of pathogen-host interactions” and her research group at the Paul-Ehrlich-Institut investigated which of the many phosphatases of the cell, which can removephosphate groups based on their enzyme activity, perform exactly this dephosphorylation at amino acid T592 of SAMHD1. It is only after dephosphorylation that SAMHD1 is antivirally active. The research team also studied how this reaction is temporally regulated during the cell cycle.
To do this, the researchers used two complementary proteomics approaches: These are procedures in which protein-related analyses are performed. The researchers studied the cell cycle and the influence of phosphorylation or dephosphorylation processes – as applicable – on the antiviral activity of SAMHD1.
In doing so, they identified the key enzyme which makes the antiviral activity of SAMHD1 possible, i.e. the phosphatase PP2A-B55alpha. This phosphatase obtained its cryptic name thanks to the fact that there are 90 enzyme variants (holoenzymes), but only this variant with the name PP2A-B55alpha which can convey SAMHD1 dephosphorylation and thus enable its antiviral activity. In addition, the researchers succeeded in discovering the time window in the cell cycle in which T592 dephosphorylation occurs, leading to reduced and/ or delayed HIV-1 replication.
While the phosphorylated SAMHD1 variant plays an important role in cell division and contributes to the defense against chronic inflammations, the molecule without this phosphate group is in a position to convey defense against viruses such as HIV or hepatitis virus.
The researchers intend to perform further studies in which they would like to clarify the way in which SAMHD1 provides a defense mechanism against HIV infection.
Original publication:
Schott K, Fuchs NV, Derua R, Mahboubi B, Schnellbächer E, Seifried J, Tondera C, Schmitz H, Shepard C, Brandariz-Nuñez, Diaz-Griffero F, Reuter A, Kim B, Janssens V, König R (2018): Dephosphorylation of the HIV-1 restriction factor SAMHD1 is mediated by PP2A-B55α holoenzymes during mitotic exit.
Nat Commun 9, Article number: 2227 (2018), Jun 8
DOI 10.1038/s41467-018-04671-1
The Paul-Ehrlich-Institut, the Federal Institute for Vaccines and Biomedicines, in Langen near Frankfurt/Main is a senior federal authority reporting to the Federal Ministry of Health (Bundesministerium für Gesundheit, BMG). It is responsible for the research, assessment, and marketing authorisation of biomedicines for human use and immunological veterinary medicinal products. Its remit also includes the authorisation of clinical trials and pharmacovigilance, i.e. recording and evaluation of potential adverse effects. Other duties of the institute include official batch control, scientific advice and inspections. In-house experimental research in the field of biomedicines and life science form an indispensable basis for the manifold tasks performed at the institute. The Paul-Ehrlich-Institut, with its roughly 800 members of staff, also has advisory functions nationally (federal government, federal states (Länder)), and internationally (World Health Organisation, European Medicines Agency, European Commission, Council of Europe etc.).
https://www.nature.com/articles/s41467-018-04671-1 – Full-Text-Article (Open Access)
https://www.pei.de/EN/information/journalists-press/press-releases/2018/10-defen… – This press release on the Paul-Ehrlich-Institut Website
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















