Creating a nanospace like no other
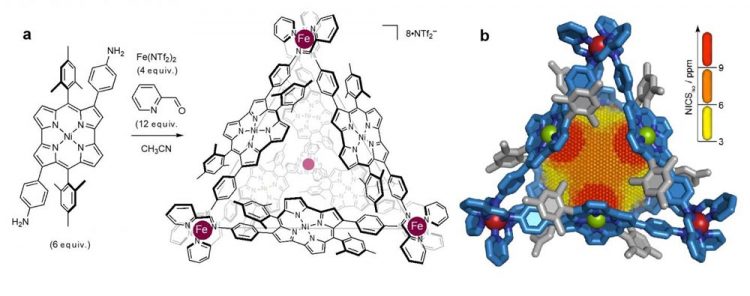
(a) construction of antiaromatic-walled nanospace. (b) X-ray crystal structure with a 3D NICS grid, showing magnetic deshielding experienced within the nanospace. Antiaromaticity effects becomes stronger in the order of yellow < orange < red color. Credit: Nature
Researchers at Tokyo Institute of Technology, the University of Cambridge, and the University of Copenhagen have built a self-assembled nanocage with a very unusual nanospace:
Its walls are made of antiaromatic molecules, which are generally considered too unstable to work with. By overturning assumptions about the limits of nano-chemical engineering, the study creates an entirely new nanospace for scientists to explore. Nanometer-sized cavities are already finding a range of useful applications in chemistry, medicine and environmental science.
Scientists including Masahiro Yamashina of Tokyo Institute of Technology (JSPS Overseas Research Fellow, at that time) and Jonathan R. Nitschke of the University of Cambridge, reporting their work in the journal Nature, describe the construction of a new type of nanospace inside “a self-assembled cage composed of four metal ions with six identical antiaromatic walls.”
Until now, many teams have developed nanocages with aromatic walls, but none with antiaromatic compounds, owing to the challenges posed by their inherent instability. Aromaticity refers to a property of ring-shaped organic compounds that makes them highly stable, whereas antiaromaticity describes compounds that are far more reactive, due to a difference in the number of so-called π-electrons shared by the ring. (For a quick summary of the differences between the two types of compounds, refer to Antiaromatic molecule displays record electrical conductance.)
The team's search for a suitable building block for their nanocage led them to a 2012 study by Hiroshi Shinokubo and co-workers in Japan. This study reported the synthesis of an unusually stable, nickel-based antiaromatic compound called norcorrole. Then, drawing on Jonathan R. Nitschke and his group's expertise in subcomponent self-assembly, the team succeeded in building a three nanometer-diameter cage with a norcorrole skeleton.
To investigate the degree of antiaromacity within the cage, the team performed nucleus-independent chemical shift (NICS) calculations. The results indicated that the norcorrole panels appear to work together to enhance antiaromacity. The NICS value was consistently high in the central part of the cage, suggesting that the panels reinforce each other.
The unique environment inside the cage was further tested by encapsulating a series of guest molecules, beginning with coronene which has been already encapsulated within the aromatic cage.
The researchers hypothesized that when exposed to an external magnetic field, guest molecules in an aromatic-walled cage would experience a shielding effect, while those in an antiaromatic-walled cage would experience a deshielding effect.
As predicted by theory, nuclear magnetic resonance (NMR) spectroscopy analyses revealed a deshielding effect attributable to the antiaromatic walls.
All guest molecules tested in the study showed significant downfield chemical shifting, an indicator of the degree of deshielding. The shift differences ranged from 0.7 to 14.9 parts per million. Of these, a carbon nanobelt showed the highest degree of downfield shifting observed so far resulting from an antiaromatic environment.
The cage can be considered as a new type of NMR shift reagent, the researchers say, meaning that it could be a useful tool for structural analysis, ie for interpreting the finest structures of organic compounds.
Future work will focus on investigating chemical reactivity within the nanospace.
###
Related links
The Nitschke Group, Department of Chemistry, University of Cambridge https:/
Department of Chemistry, School of Science https:/
Sweet success: Nanocapsule perfectly binds sucrose in water |Tokyo Tech News https:/
Stabilization of Highly Reactive Reagents upon Encapsulation |Tokyo Tech News https:/
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…

Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















