Chipping away at how ice forms could keep windshields, power lines ice-free
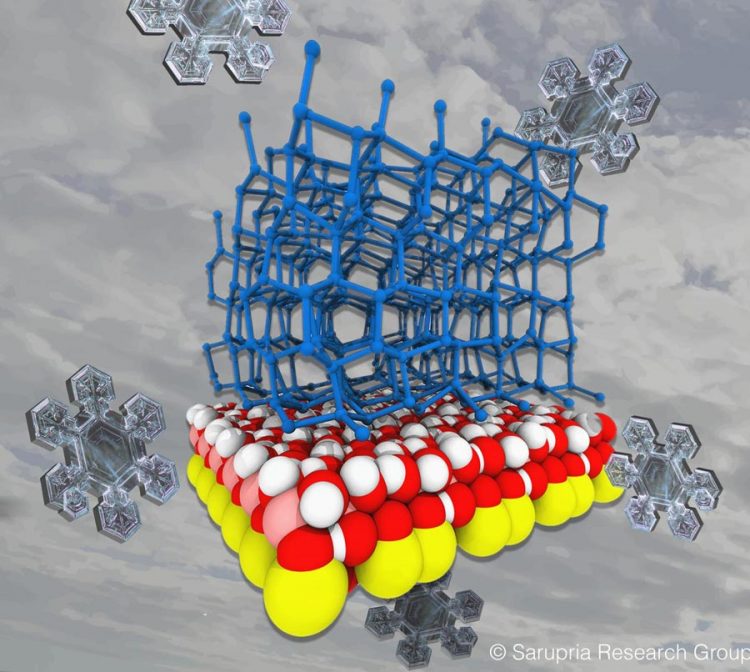
Surface atoms on a dust particle (shown in red, white, pink and yellow) impose an arrangement on water molecules (blue) that can promote or inhibit ice formation. Credit: Sapna Sarupria and Ryan DeFever
The researchers will present their findings today at the American Chemical Society (ACS) Fall 2019 National Meeting & Exposition. ACS, the world's largest scientific society, is holding the meeting here through Thursday. It features more than 9,500 presentations on a wide range of science topics.
“We discovered that if we look at the liquid water structure where it contacts the surface, we can start to understand and predict whether a given surface will promote or inhibit ice formation,” says Sapna Sarupria, Ph.D., the project's principal investigator.
“We're working with collaborators to use this information to better understand the role of ice in weather and to design surfaces that are good or bad for ice formation. Wouldn't it be great to have a windshield that doesn't let ice stick to it in winter?”
Sarupria's team uses computers to study molecular simulations of surfaces and ice formation. Unlike the messier real world, this controlled setting gives her the ability to examine the impact of a change in just one surface parameter — or even just one atom — at a time. The researchers then correlate the findings with those of experimentalists who work with real-world materials, including silver iodide or minerals such as mica and kaolinite. Silver iodide is so effective at promoting ice formation that it's used for cloud seeding to stimulate rainfall during droughts.
Ice formation, or nucleation, occurs when liquid water undergoes a phase transition to solid water. Water can also undergo other phase transitions, such as changing from ice back to a liquid, or to vapor. If these transitions take place in clouds, they can form raindrops and snow.
“When you want to predict the weather, you need to know how these phase transitions happen, and that's essentially an open question,” says Sarupria, who is at Clemson University. Often these changes occur in the presence of particles such as mineral dust in the atmosphere. The type and amount of dust determine the type of precipitation that occurs. “We're trying to understand how different dust particle surfaces affect the transition of water from the liquid to the solid phase in clouds,” she says.
Good old H2O is just that: an oxygen bound to two hydrogens. Those hydrogens are attracted to some surfaces more than others, and that affects how water molecules orient on a surface. Their arrangement with respect to surface atoms on dust particles and in relation to other water molecules is actually the most important factor in ice formation, Sarupria's team discovered.
This finding also explains why silver iodide is such a good nucleator. First, its surface atoms are laid out in a way that's similar to the arrangement of water molecules in ice, so it's an effective template. Second, the positive charge of the silver ion and negative charge of the iodine orient the hydrogens and oxygens of liquid water in the right manner for it to form an ice structure. “The distances between the atoms, and this arrangement of charges, are very important for silver iodide to be a nucleator,” Sarupria says.
The researchers are now collaborating with experimentalists who study atmospheric phenomena to help them explain their results. “If we can model these phenomena, we may be able to better understand the role of ice in weather,” she explains.
Sarupria is also applying her understanding of water structure to design surfaces that can promote or inhibit ice formation. For example, to prevent damage during food storage or cryopreservation of organs, someone in the future could use the new knowledge to form ice at temperatures closer to 32 F, the freezing point of water, rather than at lower temperatures. This could be done by modifying the surface of the packaging or adding molecules to the solution for cryopreservation.
“In other cases, such as windshields and power lines, you may not want ice to form,” Sarupria says. “So we're trying to figure out how to make coatings or surfaces that won't let ice form, or if it forms, that won't let it stick.” Her team is also trying to understand how natural antifreeze proteins help fish and other organisms survive in frigid conditions.
“Ultimately, whether it's these proteins or dust particles, it all boils down to how they affect the water structure,” she says. “We want to use this information to create a parameter that could help us quickly screen surfaces for their ice nucleation ability.”
###
A press conference on this topic will be held Tuesday, Aug. 27, at 1 p.m. Pacific time in the San Diego Convention Center. Reporters may check-in at the press center, Room 14B, Mezzanine Level, or watch live on Youtube http://bit.
The researchers acknowledge support and funding from the National Science Foundation.
The American Chemical Society, the world's largest scientific society, is a not-for-profit organization chartered by the U.S. Congress. ACS is a global leader in providing access to chemistry-related information and research through its multiple databases, peer-reviewed journals and scientific conferences. ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies. Its main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note to journalists: Please report that this research was presented at a meeting of the American Chemical Society.
Follow us on Twitter | Facebook
Title
Understanding heterogeneous ice nucleation through water structure and dynamics
Abstract
Heterogeneous ice nucleation relates to freezing of water driven by the presence of an impurity such as a mineral surface. Interestingly, it is not yet understood what about a surface promotes or inhibits the formation of ice. In our research, we evaluate the role of various surface properties on ice nucleation using molecular dynamics simulations. We have studied three types of surfaces – kaolinite-based, silver iodide-like and mica surfaces. Capitalizing on the power of molecular simulations we have carefully probed the effects of specific surface properties such as hydrogen bonding abilities, lattice spacing, and surface charge distribution on the propensity to observe ice nucleation. Furthermore, we related the structure of the metastable liquid near the surface to the propensity of ice nucleation. We find that certain characteristics of the liquid water structure – related to the orientations of water molecules near the surface – are good indicators of possible ice nucleation. We are expanding our understanding to correlate these observations with experimental findings. To this end, we focus on studies of ice nucleation near mica surfaces. Mica surfaces can be cleaved to be atomistically smooth in experiments making them an ideal choice for a collaborative experimental and simulation investigation. We study the effects of surface ion and charge distribution on ice nucleation. This combined approach provides information regarding the molecular mechanisms of ice nucleation near mica surfaces and the effect of surface ions on this behavior. Collectively, we focus on understanding the hydration behavior of water near the surfaces and its relation to likelihood of observing ice nucleation at these surfaces. Such insights help design surfaces that either inhibit ice nucleation (e.g., in cases such as wind mills, power lines, and transportation) or that promote ice nucleation (e.g., in cases such as food preservation and cryopreservation).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















