Catalyst structure identified in an operating PEM fuel cell
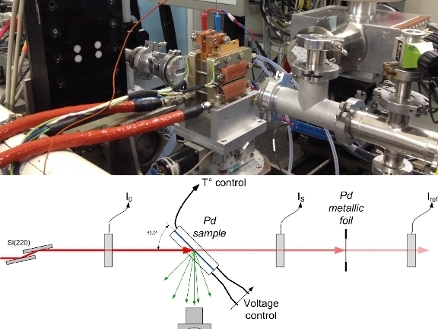
Photograph and schematic lay-out of the experimental setup, featuring an improved flow field design and a reduced thickness of the graphite window (500 µm). The latter is transparent to X-rays at the K edge energies (6 8 keV) of transition metal catalysts such as manganese, iron, cobalt and nickel. The improved cell design therefore also enables operando XAS studies of commonly investigated fuel cell catalysts based on these 3d transition metal alloys with platinum, or of PGM-free iron-based catalysts.
The research, performed in collaboration with the Technical Electrochemistry research group of Prof. Dr Hubert Gasteiger at the Technical University of Munich (Department of Chemistry), has recently been published in ACS Catalysis.
Investigating palladium
In proton exchange membrane fuel cells (PEMFC) electrons are generated by means of the electrochemical oxidation of hydrogen, thus producing the electrical power to drive an electric car or provide electricity for industry or households. The best currently known electro-catalysts for this electron-generating oxidation reaction are the so-called platinum-group metals, with platinum itself as the most active catalyst.
Palladium provides an interesting alternative for platinum since it is only slightly less active but more widely available and less expensive. However, in practice the activity of palladium decreases at high anodic potentials. This has until now been explained by a change in its catalytic properties, mainly hydride decomposition in the bulk of the material and oxide formation at the surface.
These explanations are disputable, however, since they are based on laboratory experiments at room temperature. Typical operating conditions of a low temperature PEMFC involve temperatures up to 80 °C. Both for a fundamental understanding of the performance and for the development of non-Pt based catalysts it is important to characterize the catalyst under real reaction conditions.
Improved experimental set-up
The current Amsterdam/Munich research cooperation bridges the gap between electrochemical studies in liquid electrolytes at room temperature and real operating fuel cells at 80 °C. In ACS Catalysis the researchers present electrochemical isotherms for the absorption of hydrogen into a Pd catalyst as a function of applied potential, temperature, and reaction atmosphere.
They were obtained with a new, improved X-ray absorption spectroscopy (XAS) electrochemical fuel cell, allowing the investigation of PEMFC electrodes during operation (operando spectroscopy). The research was performed at the BM30B/FAME beamline of the European Synchrotron Radiation Facility in Grenoble.
Hydride phase maintained
The operando spectroscopic characterization during hydrogen oxidation unequivocally demonstrates that the hydride phase is maintained under practical operating conditions of a fuel cell anode, even at high anodic potentials. The transition from a hydride to a metallic state, previously observed in electrochemical cells based on a liquid electrolyte, does not occur.
The researchers argue that the reaction environment of operating PEMFC's is so much unlike that in room-temperature liquid electrolytes cells that the chemical state of the Pd catalyst is completely different. One important feature explaining this is the orders of magnitude higher mass-transport rates in PEMFC's.
The recent findings highlight the necessity of characterizing the properties of electro-catalysts under realistic operating conditions. Furthermore the researchers argue that in fact for all electro-catalytic reactions in which the reactant is supplied in a gaseous form – not just for the hydrogen oxidation in a fuel cell – it is of utmost importance to maintain appropriate mass transfer regimes when establishing structure-activity relationships.
Full bibliographic information
Armin Siebel, Yelena Gorlin, Julien Durst, Olivier Proux, Frédéric Hasché, Moniek Tromp, and Hubert A. Gasteiger: Identification of Catalyst Structure during the Hydrogen Oxidation Reaction in an Operating PEM Fuel Cell ACS Catal., 2016, 6, pp 7326–7334 DOI: 10.1021/acscatal.6b02157
For further information, please contact:
Johan Rheeder
+31 20 525 3591
Media Contact
More Information:
http://www.uva.nlAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















