The blue blood of the emperor scorpion x-rayed
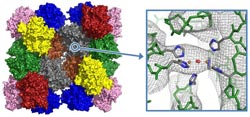
Hemocyanin of the emperor scorpion: model of the 24-meric protein complex and electron density at the active site where oxygen binding takes place.<br>Abb./©: E. Jaenicke et al (2012), PLoS One 7(3):e32548<br>
The emperor scorpion (Pandinus imperator) is not only one of the biggest scorpions in the world, but it also has one remarkably large protein, namely hemocyanin. Hemocyanin is a protein complex made up of 24 subunits that functions as blood pigment. It is one of the largest known proteins, comparable in size to ribosomes or even small viruses.
For the first time ever, scientists from Johannes Gutenberg University Mainz in Germany have now successfully grown crystals from the emperor scorpion’s hemocyanin. With the help of x-rays, these crystals allow for a more precise analysis of the structure of the protein. Up to now, cryo-electron microscopy has primarily been used to examine large protein structures such as hemocyanin.
This method has its disadvantages, however, because its resolution is not sufficient to be able to differentiate between single atoms. With x-ray crystallography, on the other hand, protein structure can be more precisely determined. It is even possible to determine the spatial arrangement of individual atoms. Scientists rely on this knowledge about the detailed molecular structure of these protein complexes in order to be able to understand how these proteins function.
Hemocyanins are extraordinarily large respiratory proteins that transport oxygen in the blood of mollusks and arthropods. While these blue blood proteins bind oxygen between two copper atoms, human hemoglobin binds oxygen to iron atoms. Hemocyanin fascinates biologists because, depending on the animal species, up to 160 oxygen binding sites within a single protein complex must communicate with one another in order to bind, transport, and release oxygen in the blood. Referred to as cooperativity, this phenomenon occurs only in nature and could potentially be used in nanotechnology applications to build molecular switches. Structure determination at an atomic resolution is necessary in order to be able to understand this process in detail.
For the first time ever, Professor Dr. Elmar Jaenicke from the Institute of Molecular Biophysics at Johannes Gutenberg University Mainz has managed to crystallize the blue hemocyanin protein complex from the emperor scorpion. This is the decisive first step toward successful x-ray structure determination because protein crystals are necessary to diffract x-rays so that the structure of the protein can be determined. Crystallization, however, is especially difficult for large protein complexes. “It is a little bit like a game of chance,” Jaenicke describes the crystallization process, because the process is dependent on a number of factors such as the pH-level, the salinity of the solution, or the temperature. “The decisive step is always crystal nucleation,” which, according to Jaenicke, can take months and requires a lot of patience. Sometimes, it even takes several years to optimize the conditions for crystallization. This is the reason why so far only a handful of molecular structures of very large protein complexes have been solved using x-ray structure determination worldwide. In fact, one of these structural analyses – namely that of the ribosome – was awarded the Nobel Prize in 2009.
The crystals are measured in the x-ray beam, and the structure is then determined through complex calculations based on the scattered x-rays. At first, Jaenicke and his team of scientists were able to attain a mid-resolution (6.5 ångströms) structure for the emperor scorpion's protein with which secondary structures such as α-helices could be seen, but other elements, such as single amino acids, could not yet be ascertained. In layman's terms: If the protein is a brick house and a telescope is used to try to look at its structure from far away, the windows, doors, and the mailbox would be visible at the current resolution, but the arrangement of the individual bricks would not. “This was our starting point and now we can already see parts of the active site of the molecule. With further improvements to our crystals, we are well on our way to achieving an atomic resolution that is not possible with any other method.” According to Jaenicke, the oxygen binding protein from the emperor scorpion would then be one of the five largest structures to have been deciphered using x-ray structure analysis to date.
Johannes Gutenberg University Mainz has the ideal infrastructure to support this type of structural research on very large protein complexes which can only be done at a few research institutes around the world. In Mainz, x-ray structure determination projects in the research groups of Professor Dr. Heinz Decker and Professor Dr. Elmar Jaenicke at the Institute of Molecular Biophysics cover the atomic resolution range, cryo-electron microscopy studies in the research group of Professor Dr. Jürgen Markl at the Institute of Zoology take care of the mid-resolution range. The new rotating anode x-ray generator used at the Institute of Molecular Biophysics is also ideal for determination of the structure of these giant molecules because it produces focused x-ray beams with an intensity comparable to that of second-generation synchrotron beamlines.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















