Architecture of mTOR Protein Complex Solved
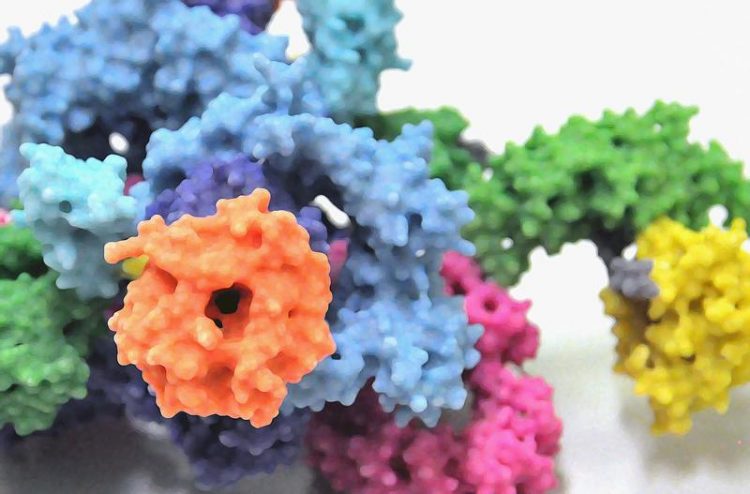
3D model of the protein complex mTORC1. University of Basel, Biozentrum
About 25 years ago, Prof. Michael Hall discovered the protein “Target of Rapamycin” (TOR) at the Biozentrum. It is one of the most studied proteins of the protein kinase family, an important family of regulatory proteins that control many cellular processes.
TOR, in mammals called mTOR, is very important for cellular signalling and is implicated in various diseases such as cancer, diabetes, and neurodegeneration. Several mTOR inhibitors have already been approved for therapeutic use, in particular in the treatment of cancer and allograft rejection.
However, despite extensive research on TOR over the last decades, attempts to uncover the detailed structure of the protein kinase and its partners have been unsuccessful. By combining crystallographic methods with cryo-electron microscopy, Prof. Timm Maier’s team together with researchers of the ETH Zurich have now been able to provide unprecedented insight into the architecture of the protein complex mTORC1.
Structure of mTORC1 elucidated
In the cell, the protein kinase mTOR is found in two structurally and functionally distinct protein complexes termed mTORC1 and mTORC2 in mammals. Both complexes are giant protein structures consisting of mTOR and other accompanying proteins. In these two configurations the protein kinase carries out various functions such as the control of cell size and growth, as well as the regulation of metabolism and energy balance.
mTOR itself is one of the largest proteins in the cell and when combined with other proteins even larger. This makes it quite difficult to investigate its structure. “The partner proteins of mTOR have already been identified in earlier biochemical studies”, says Maier.
“However, it has remained unclear how the proteins interact precisely.” After more than three years of work, the scientists led by Maier have succeeded in isolating mTORC1 in the quality required for high-resolution cryo-electron microscopy. Using X-ray crystallography they have also been able to determine the structure of the protein Raptor, the second major component of mTORC1.
Accompanying proteins important for function
“Although there is much known about mTORC1, our study revealed surprising new insight”, states Maier. “The architecture of this huge protein complex is quite exceptional. We could determine the precise interaction sites of the partner proteins and how they are arranged, and thus elucidate the function of the individual partners.” In fact, each protein plays an important role in the regulation of the activity of the entire complex and the intracellular signalling cascade.
More than the sum of its parts
With their study, the researchers have provided the basis for further investigations. Now the researchers will be able to investigate the function of each individual protein in the complex in more detail. “But it doesn’t make sense to examine the individual components alone, as the interactions of all the proteins in the complex are critical for its function”, explains Maier. “The whole is much more than the sum of its parts.” The finely tuned regulation of mTOR activity is very important because even the smallest disturbances can have serious consequences. Thus, dysregulation in the mTOR signalling pathways plays a role in the development of a number of diseases.
Original source
Christopher H.S. Aylett, Evelyn Sauer, Stefan Imseng, Daniel Boehringer, Michael N. Hall, Nenad Ban and Timm Maier
Architecture of Human mTOR Complex 1
Science, published online 17 December 2015.
Further information
Prof. Dr. Timm Maier, University of Basel, Biozentrum, tel. +41 61 267 21 76, email: timm.maier@unibas.ch
Dr. Katrin Bühler, University of Basel, Biozentrum, Communications, tel. +41 61 267 09 74, email: katrin.buehler@unibas.ch
https://www.unibas.ch/en/News-Events/News/Research/Architecture-of-mTOR-Protein-…
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















