Acrobatic duo in the cells
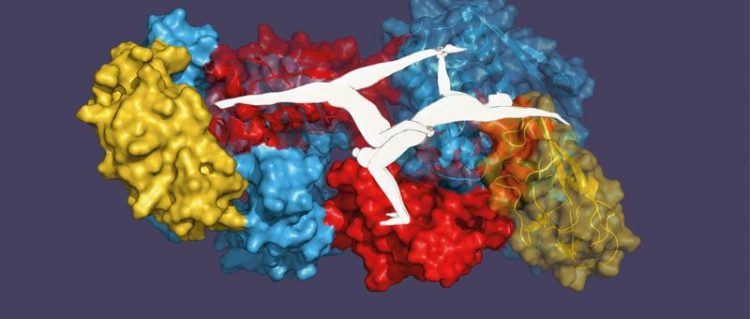
Like an acrobatic duo – single proteins lend each other greater stability.
Misfolded proteins are non-functional and cause cell damage. In order to prevent this, there is a whole arsenal of proteins – called chaperones – that assist with folding and carry out quality control. In the bacterium Escherichia coli, the chaperone “Trigger factor” (TF) protects the newly produced proteins from misfolding.
Prof. Sebastian Hiller’s research group at the Biozentrum of the University of Basel has now shown for the first time that TFs also recognize and stabilize each other. Just like single acrobats from a duo, single TF chaperones stand on shaky legs. Only as a pair they find a stable position.
Chaperones help folding of other proteins
Within one single bacterial cell, more than 10,000 ribosomes produce proteins non-stop. These factories link the individual protein components to form a long peptide chain and transport it outwards through a narrow channel.
The chaperone TF, which is bound to the tunnel exit of the ribosome, receives the freshly assembled protein and, while shielding it from the environment, helps it to fold correctly. When the protein has found its correct spatial structure, it is released from the chaperone and can get on with its work in the cell.
Whether acrobat or chaperone – stability only as a duo
In the cell, there are considerably more TF proteins than ribosomes. This ensures that the X-thousand ribosomes are fully occupied and that each of the newly produced proteins can be collected. The surplus TF proteins, like acrobatic pairs, join with a partner to form a stable duo. The pairing happens completely on its own.
“In unpaired TF proteins the region that would bind to the ribosome is folded unfavorably and therefore energetically unstable,” explains Hiller. “In the search for an energetically favorable, stable structure, this labile domain is continuously reoriented. TFs are able to detect such dynamic regions of a protein, also among each other.” In combining, the two instable TF proteins, like two acrobats connecting at the crucial point, form a stable spatial arrangement.
Chaperones detect dynamic protein domains
“The latest findings about the dynamics of stable ‘TF-duos’ make it possible to draw important conclusions about the functioning of chaperones. Upon recognition, they do not form just one type of protein structure but rather a dynamic ensemble of different spatial arrangements,” says Hiller. “It is becoming apparent that this functionality is a general pattern for chaperones.”
The elucidation and understanding of the chaperone function at the atomic level is important to the research community worldwide. Problems in the folding process of proteins are associated with various diseases such as the metabolic disorder Cystic fibrosis, cancer or Alzheimer’s disease.
Original article
Leonor Morgado, Björn M. Burmann, Timothy Sharpe, Adam Mazur, Sebastian Hiller
The dynamic dimer structure of the chaperone Trigger Factor
Nature Communications (2017), doi: 10.1038/s41467-017-02196-7
Further information
Prof. Dr. Sebastian Hiller, University of Basel, Biozentrum, tel. +41 61 207 20 82, email: sebastian.hiller@unibas.ch
Dr. Katrin Bühler, University of Basel, Biozentrum, Communications, Tel. +41 61 207 09 74, email: katrin.buehler@unibas.ch
Media Contact
More Information:
http://www.unibas.chAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















