A vocabulary of ancient peptides
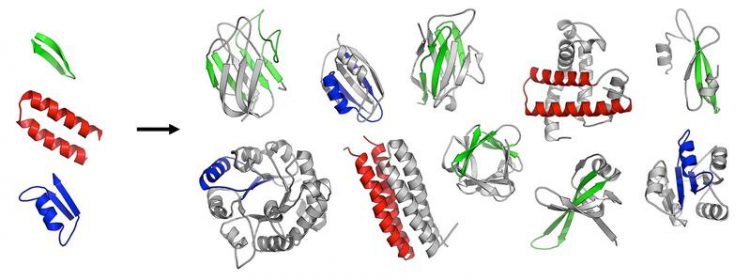
The first folded proteins seem to have originated from an ancestral set of peptides. © MPI for Developmental Biology/ Vikram Alva
Proteins and languages share many similarities – both, for instance, yield their meaning through a proper arrangement of basic building blocks. Andrei Lupas, Director at the Max Planck Institute for Developmental Biology in Germany, and his team apply computational methods to reconstruct primordial building blocks by comparative studies of modern proteins.
The same approach is used in linguistics to reconstruct ancient vocabularies through the comparison of modern languages. In a recent study the scientists report the identification of 40 ancestral, peptidic fragments, which possibly represent the observable remnants of a time when the first proteins were created, more than 3.5 billion years ago.
Proteins are integral building blocks of all life, from bacteria to humans. In our bodies, they are essential for all chemical processes: they form our nails, hair, bones, and muscles, they help digest the food we eat, and they defend us form pathogenic bacteria and viruses.
“Life can be viewed as substantially resulting from the chemical activity of proteins”, says Lupas, Director of the Department of Protein Evolution at the Max Planck Institute for Developmental Biology. He and his collaborators are particularly interested in understanding how these complex biomolecules originated.
Today we know that proteins are primarily built through the combinatorial assembly of only a few thousand modular units, termed domains. It is however unclear how these modular units themselves emerged.
The scientists investigated the hypothesis that the first protein domains arose by fusion and piecemeal growth from an ancestral set of simple peptides, which themselves emerged in an RNA-based pre-cellular life, around 3.5 billion years ago.
In a systematic analysis of modern proteins, they were able to identify 40 peptidic fragments that occur in seemingly unrelated proteins, yet bear striking resemblance in their sequences and structures. Based on their widespread occurrence in the most ancient proteins (e.g., ribosomal proteins) and on their involvement in basal functions (e.g., RNA-binding, DNA-binding), the authors propose that these fragments are the observable remnants of a primordial RNA-peptide world, a precursor form of the DNA-based life we know today.
In the future, the contribution of these fragments to the formation of protein structure will have to be investigated experimentally, opening new avenues to optimize existing proteins and design new ones, not yet seen in nature. “If we elucidate this process, we should be able to create new protein forms”, concludes Lupas, with exciting applications to biotechnology.
Contact
Prof. Dr. Andrei N. Lupas
Max Planck Institute for Developmental Biology, Tübingen
Phone: +49 7071 601-340
Fax: +49 7071 601-349
Email: andrei.lupas@tuebingen.mpg.de
Nadja Winter
Max Planck Institute for Developmental Biology, Tübingen
Phone: +49 7071 601-444
Fax: +49 7071 601-446
Email: presse-eb@tuebingen.mpg.de
Original publication
Alva V, Söding J, Lupas AN
A vocabulary of ancient peptides at the origin of folded proteins.
Elife. 2015 Dec 14;4. pii: e09410. doi: 10.7554/eLife.09410
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















