A cancer protein’s journey to cell membrane
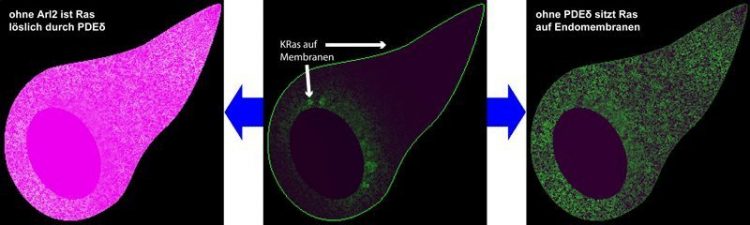
Distribution of the cancer protein KRas in a cell. © M. Schmick
The cancer protein KRas is a factor in the development of several types of cancer. Mutated KRas, for example, can be found in a large number of all tumour cells in patients with pancreatic cancer. It sits on the inner leaflet of the cell membrane and relays signals into the cell’s interior.
Scientists at the Max Planck Institute of Molecular Physiology in Dortmund have now discovered why KRas is almost exclusively found at the cell membrane when observed under the microscope. Apparently, the protein is not specifically sent to the cell membrane after it is formed, but is also located on other membrane systems within the cell for its entire life span.
In order for it to be transported by special transport vesicles from the vicinity of the cell nucleus to the cell membrane, the solubilising factor PDEδ and its antagonist Arl2 must be active. Without the two of them, KRas would spread to cell membranes. The researchers can use their results to better understand how deltarasin works, which is a potential anti-cancer drug that they have developed.
The protein KRas acts as a molecular switch in relaying signals to the cell’s interior. Among other things, such signals control cell growth. In order for KRas to be able to function correctly, it must remain on the inner leaflet of the cell membrane for a sufficient period of time. Its water-insoluble lipid anchor helps it to achieve this.
However, this also anchors the protein to other intracellular membranes. KRas therefore has an area of positive charges near this lipid anchor. Similar to a polystyrene ball in a plastic bag, the electrostatic interaction of these positive charges with the negatively charged inner leaflet of the cell membrane reinforces the lipid anchoring.
But even lipid anchor and positive charges are not enough to ensure that KRas is permanently enriched at the cell membrane. According to the results obtained by the researchers in Dortmund, many KRas molecules would still be lost on the available surface of the rest of the membrane systems in the cell, which is 200 times bigger than that of the cell membrane. Using complex computer simulations, the scientists evaluated data from fluorescence microscopy experiments and tracked the movement of KRas on its journey though the cell.
“Our results show that the cell membrane is by no means the final destination of KRas, which must only be encountered once. Instead, KRas constantly and unspecifically re-distributes to the various membrane systems of the cell and must then be concentrated on the inner leaflet of the cell membrane via a continuous cycle,” explains Malte Schmick from the Max Planck Institute of Molecular Physiology.
In the first step of this cycle, the soluble protein PDEδ shields the lipid anchor of KRas like a glove, thus making KRas water-soluble. This prevents KRas from simply finding some arbitrary membrane. Swimming in the cytoplasm, KRas can thus explore the cell. When it gets close to the nucleus of the cell, the activity of the protein Arl2 removes this glove. KRas is now insoluble in water again and can be trapped on membranes and transported back to the cell membrane by vesicles.
The cell therefore does not have a unique targeting system for KRas, which sends it exclusively to the cell membrane. Instead, the protein redistributes to all membranes and is repeatedly sorted from the wrong membranes to the correct one. “Each KRas molecule lives for several hours before the cell disassembles it again. After seven minutes, half of all KRas molecules are internalized from the cell membrane to be subjected to the cycle and sent back to the cell membrane. In total, each KRas molecule travels for approximately 20 minutes before it reaches the cell membrane again,” says Schmick.
The results obtained by the scientists in Dortmund pave the way for the development of new cancer drugs. This is due to the fact that KRas is modified in many forms of cancer to such an extent that it is permanently active and the cell can no longer switch it off. One-third of all tumours contain cells with mutations of Ras proteins. In the case of intestinal cancer for example, mutated KRas prevents successfully using antibody treatment against epidermal growth factor receptors (EGFR).
“We can now develop active agents that reduce the enrichment of mutated, permanently active KRas at the cell membrane,” explains Philippe Bastiaens, Director at the Max Planck Institute in Dortmund. In 2013, he worked with colleagues Herbert Waldmann and Alfred Wittinghofer to develop an inhibitor, known as deltarasin, to block the PDEδ solubilizing activity. Initial experiments on mice have shown that the active agent considerably slows the growth of tumours. Even though scientists had been aware of the relevance of PDEδ for a while, this work now explains for the first time the mechanism by which deltarasin prohibits KRas from being enriched at the cell membrane and causes it to be distributed throughout the entire cell.
Contact
Fax: +49 231 1332-299 Email:philippe.bastiaens@mpi-dortmund.mpg.de
Fax: +49 231 133-2599
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…

A flexible and efficient DC power converter for sustainable-energy microgrids
A new DC-DC power converter is superior to previous designs and paves the way for more efficient, reliable and sustainable energy storage and conversion solutions. The Kobe University development can…
Technical Trials for Easing the (Cosmological) Tension
A new study sorts through models attempting to solve one of the major challenges of contemporary cosmic science, the measurement of its expansion. Thanks to the dizzying growth of cosmic…





















