30 years after C60: Fullerene Chemistry with Silicon
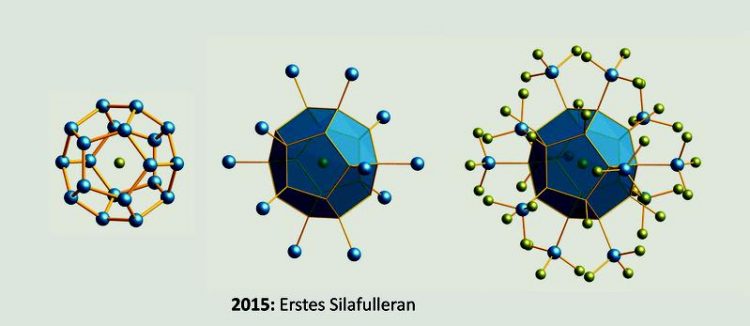
Silafulleran GU
The discovery of the soccer ball-shaped C60 molecule in 1985 was a milestone for the development of nanotechnology. In parallel with the fast-blooming field of research into carbon fullerenes, researchers have spent a long time trying in vain to create structurally similar silicon cages.
Goethe University chemists have now managed to synthesise a compound featuring an Si20 dodecahedron. The Platonic solid, which was published in the “Angewandte Chemie” journal, is not just aesthetically pleasing, it also opens up new perspectives for the semiconductor industry.
The Si20 dodecahedron is roughly as large as the C60 molecule. However, there are some crucial differences between the types of bonding: All of the carbon atoms in C60 have a coordination number of three and form double bonds. In the silicon dodecahedron, in contrast, all atoms have a coordination number of four and are connected through single bonds, so that the molecule is also related to dodecahedrane (C20H20).
“In its day, dodecahedrane was viewed as the 'Mount Everest' of organic chemistry, because it initially could only be synthesized through a 23- step sequence. In contrast, our Si20 cage can be created in one step starting from Si2 building blocks,” explains Prof. Matthias Wagner of the Goethe University Institute of Inorganic and Analytical Chemistry.
The Si20 hollow bodies, which have been isolated by his PhD student, Jan Tillmann, are always filled with a chloride ion. The Frankfurt chemists therefore suppose that the cage forms itself around the anion, which thus has a structure-determining effect. On its surface, the cluster carries eight chlorine atoms and twelve Cl3Si groups.
These have highly symmetric arrangements in space, which is why the molecule is particularly beautiful. Quantum chemical calculations carried out by Professor Max C. Holthausen's research group at Goethe University show that the substitution pattern that was observed experimentally indeed produces a pronounced stabilisation of the Si20 structure.
In future, Tillmann and Wagner are planning to use the surface-bound Cl3Si anchor groups to produce three dimensional nanonetworks out of Si20 units. The researchers are particularly interested in the application potential of this new compound:
“Spatially strictly limited silicon nanoparticles display fundamentally different properties to conventional silicon wafers,” explains Matthias Wagner. The long strived-for access to siladodecahedrane thus opens up the possibility of studying the fundamental electronic properties of cage-like Si nanoparticles compared to crystalline semiconductor silicon.
Publication
J. Tillmann et al: One-Step Synthesis of a [20]Silafullerane with an Endohedral Chloride
Ion, in: Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201412050
Download an image from: http://www.muk.uni-frankfurt.de/54612947?
Information: Prof. Matthias Wagner, Institute for Anorganic and Analytic Chemistry, Campus Riedberg, phone: +49(069)798-29156, email: Matthias.Wagner@chemie.uni-frankfurt.de
Goethe University is a research-oriented university in the European financial centre Frankfurt Founded in 1914 with purely private funds by liberally-oriented Frankfurt citizens, it is dedicated to research and education under the motto “Science for Society” and to this day continues to function as a “citizens’ university”. Many of the early benefactors were Jewish. Over the past 100 years, Goethe University has done pioneering work in the social and sociological sciences, chemistry, quantum physics, brain research and labour law. It gained a unique level of autonomy on 1 January 2008 by returning to its historic roots as a privately funded university. Today, it is among the top ten in external funding and among the top three largest universities in Germany, with three clusters of excellence in medicine, life sciences and the humanities.
Publisher The President of Goethe University, Marketing and Communications Department, 60629 Frankfurt am Main
Editor: Dr. Anke Sauter, Science Editor, International Communication, Tel: +49(0)69 798-12498, Fax +49(0)69 798-761 12531, sauter@pvw.uni-frankfurt.de
Internet: www.uni-frankfurt.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















