Sandia uses confined nanoparticles to improve hydrogen storage materials performance
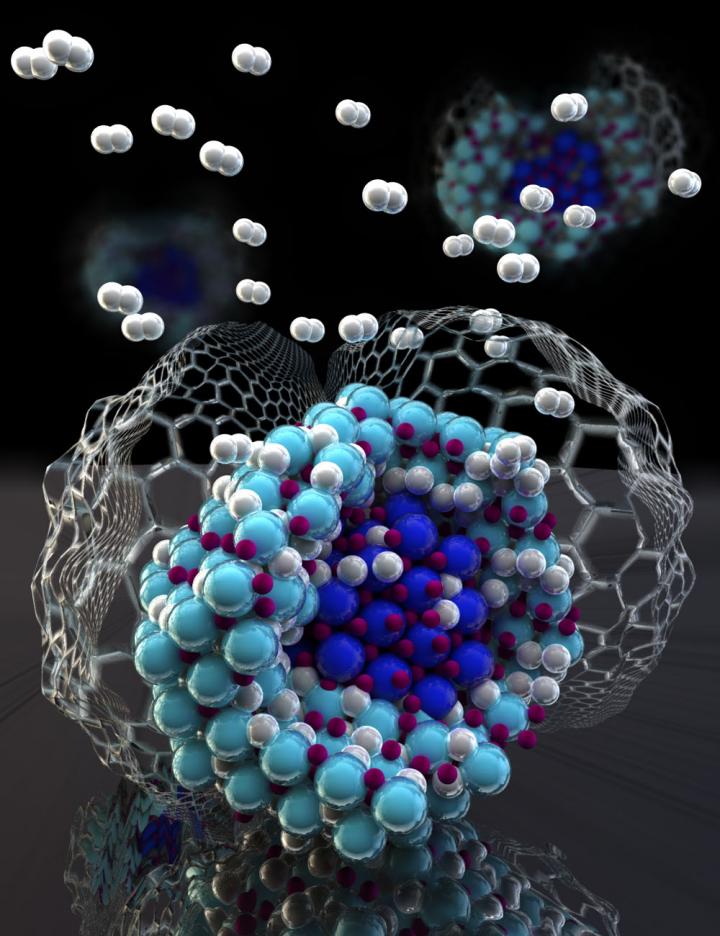
Hydrogenation forms a mixture of lithium amide and hydride (light blue) as an outer shell around a lithium nitride particle (dark blue) nanoconfined in carbon. Nanoconfinement suppresses all other intermediate phases to prevent interface formation, which has the effect of dramatically improving the hydrogen storage performance. Credit: Sandia National Laboratories
Sometimes, you have to go small to win big. That is the approach a multilab, interdisciplinary team took in using nanoparticles and a novel nanoconfinement system to develop a method to change hydrogen storage properties. This discovery could enable the creation of high-capacity hydrogen storage materials capable of quick refueling, improving the performance of emerging hydrogen fuel cell electric vehicles. Sandia National Laboratories, Lawrence Livermore National Laboratory (LLNL), the National Institute of Standards and Technology and Mahidol University in Bangkok, Thailand, collaborated on the research, which was published Feb. 8 in the journal Advanced Materials Interfaces.
The work was funded by the Department of Energy's (DOE) Fuel Cell Technologies Office and the Boeing Co.
Accelerating the uptake and release of hydrogen
Hydrogen fuel cell vehicles are powered by an electrochemical reaction between hydrogen and oxygen inside a fuel cell. While oxygen is provided by air, the hydrogen must be stored separately on the vehicle. Current fuel cell electric vehicles store hydrogen as a high-pressure gas.
A solid material can act like a sponge for the absorption and release of hydrogen, in chemical terms hydrogenation and dehydrogenation. Thus using such a hydrogen storage material could increase how much hydrogen can be stored. The material must be able to store enough hydrogen for the vehicle to go at least 300 miles before refueling.
“There are two critical problems with existing sponges for hydrogen storage,” said Sandia chemist Vitalie Stavila. “Most can't soak up enough hydrogen for cars. Also, the sponges don't release and absorb hydrogen fast enough, especially compared to the 5 minutes needed for fueling.”
In this effort, Stavila explained, the interdisciplinary team of scientists worked closely on the synthesis, characterization and modeling to improve the properties of lithium nitride, a promising hydrogen storage sponge. The team also developed a fundamental understanding of why nanosizing improves the hydrogen storage properties of this material.
Confining the space
The idea came from Mahidol University graduate student Natchapol “Golf” Poonyayant, who approached Sandia with the idea of using nanoconfinement to enhance hydrogen storage reactions in nitrogen-containing compounds. Working with the Sandia researchers, Poonyayant, his adviser, Pasit Pakawatpanurut, and fellow Mahidol student Natee “Game” Angboonpong found that liquid ammonia could be used as a gentle and efficient solvent for introducing metals and nitrogen into the pockets of carbon nanoparticles, producing nanoconfined lithium nitride particles.
The new material that emerged from Poonyayant's idea showed some unusual and unexpected properties. First, the amount of lithium nitride in the carbon nanoparticle host was quite high for a nanoconfined system, about 40 percent. Second, the nanoconfined lithium nitride absorbed and released hydrogen more rapidly than the bulk material. Furthermore, once the lithium nitride had been hydrogenated, it also released hydrogen in only one step and much faster than the bulk system that took two steps.
“In other words, the chemical pathways for both hydrogen absorption and release in this hydrogen storage material were dramatically changed for the better,” said Sandia chemist Lennie Klebanoff.
Understanding the puzzle
To better understand the mechanism responsible for this improvement, the Sandia scientists reached out to computational scientist Brandon Wood of LLNL, a leading expert in the theory of solid-state reactions. Wood and his LLNL colleagues Tae Wook Heo, Jonathan Lee and Keith Ray discovered that the reason for the unusual behavior was the energy associated with two material interfaces.
Since the lithium nitride nanoparticles are only 3 nanometers wide, even the smallest energetically unfavorable process is avoided in the hydrogen storage properties. For lithium nitride nanoparticles undergoing hydrogenation reactions, the avoidance of unfavorable intermediates — extra steps in the chemical process — increases efficiency.
Taking the path of least resistance, the material undergoes a single-step path to full hydrogenation. Similarly, once hydrogenated, the nanoparticles release hydrogen by the lowest energy pathway available, which in this case is direct hydrogen release back to lithium nitride.
“In this way, the nanointerfaces drive the hydrogen storage properties when the materials are made very small, for example with nanoconfinement,” said Wood. “The purposeful control of nanointerfaces offers a new way to optimize hydrogen storage reaction chemistry.”
The next step
According to the Sandia and LLNL researchers, the next step is to further understand how the dehydrogenated and hydrogenated phases of lithium nitride change at the nanoscale. This is a stiff challenge to the team, as it requires imaging different chemical phases within a particle that is only several nanometers wide.
The team will draw on the capabilities within the DOE's Hydrogen Storage Materials Advanced Research Consortium (HyMARC), led by Sandia and comprised additionally of scientists from LLNL and Lawrence Berkeley National Laboratory. The team plans to use spatially resolved synchrotron radiation from LBNL's Advanced Light Source to probe interface chemistry and structure.
In addition, since the nanoporous carbon host is “dead weight” from a hydrogen storage perspective, the team is examining ways to “lighten the load” and find carbon materials with more nanopockets for a given carbon mass.
“We are thrilled with this technical advance and excited to take on the work ahead,” said Klebanoff. “But it's bittersweet. Golf, who inspired this work and conducted many of the syntheses, died tragically at the age of 25 during the writing of this paper. The world has lost a talented young man and we have lost a dear friend whom we miss. This work and its published account are dedicated to Golf and his family.”
###
Sandia National Laboratories is a multi-mission laboratory operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corp., for the U.S. Department of Energy's National Nuclear Security Administration. With main facilities in Albuquerque, N.M., and Livermore, Calif., Sandia has major R&D responsibilities in national security, energy and environmental technologies and economic competitiveness.
Media Contact
All latest news from the category: Interdisciplinary Research
News and developments from the field of interdisciplinary research.
Among other topics, you can find stimulating reports and articles related to microsystems, emotions research, futures research and stratospheric research.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…