Modelling leads to the optimum size for platinum fuel cell catalysts: Activity of fuel cell catalysts doubled
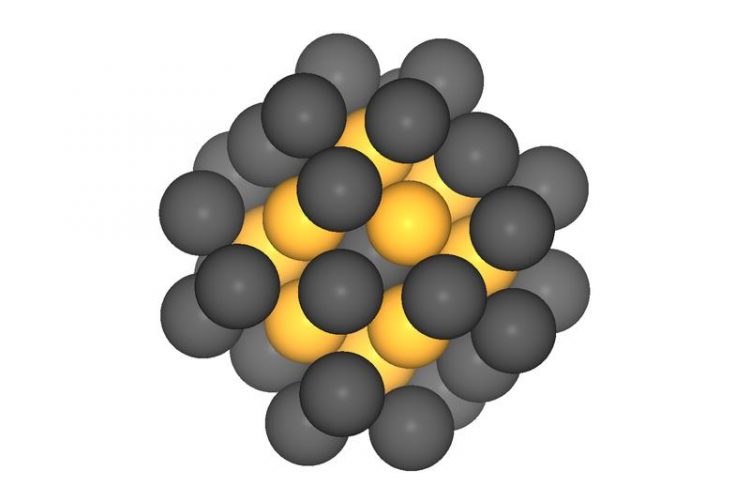
40-atom platinum nanoparticles should have the optimal catalytic effect in fuel cells. Measurements at the TUM's Catalysis Research Center confirmed the prognosis. Image: Batyr Garlyyev / TUM
Fuel cells may well replace batteries as the power source for electric cars. They consume hydrogen, a gas which could be produced for example using surplus electricity from wind power plants. However, the platinum used in fuel cells is rare and extremely expensive, which has been a limiting factor in applications up to now.
A research team at the Technical University of Munich (TUM) led by Roland Fischer, Professor for Inorganic and Organometallic Chemistry, Aliaksandr Bandarenka, Physics of Energy Conversion and Storage and Alessio Gagliardi, Professor for Simulation of Nanosystems for Energy Conversion, has now optimized the size of the platinum particles to such a degree that the particles perform at levels twice as high as the best processes commercially available today.
Ideal: A platinum egg only one nanometer long
In fuel cells, hydrogen reacts with oxygen to produce water, generating electricity in the process. Sophisticated catalysts at the electrodes are required in order to optimize this conversion. Platinum plays a central role in the oxygen-reduction reaction.
Searching for an ideal solution, the team created a computer model of the complete system. The central question: How small can a cluster of platinum atoms be and still have a highly active catalytic effect? “It turns out that there are certain optimum sizes for platinum stacks,” explains Fischer.
Particles measuring about one nanometer and containing approximately 40 platinum atoms are ideal. “Platinum catalysts of this order of size have a small volume but a large number of highly active spots, resulting in high mass activity,” says Bandarenka.
Interdisciplinary collaboration
Interdisciplinary collaboration at the Catalysis Research Center (CRC) was an important factor in the research team's results. Combining theoretical capabilities in modelling, joint discussions and physical and chemical knowledge gained from experiments ultimately resulted in a model showing how catalysts can be designed with the ideal form, size and size distribution of the components involved.
In addition, the CRC also has the expertise needed to create and experimentally test the calculated platinum nano-catalysts. “This takes a lot in terms of the art of inorganic synthesis,” says Kathrin Kratzl, together with Batyr Garlyyev and Marlon Rück, one of three lead authors of the study.
Twice as effective as the best conventional catalyst
The experiment exactly confirmed the theoretical predictions. “Our catalyst is twice as effective as the best conventional catalyst on the market,” says Garlyyev, adding that this is still not adequate for commercial applications, since the current 50 percent reduction of the amount of platinum would have to increase to 80 percent.
In addition to spherical nanoparticles, the researchers hope for even higher catalytic activity from significantly more complex shapes. And the computer models established in the partnership are ideal for this kind of modelling. “Nevertheless, more complex shapes require more complex synthesis methods,” says Bandarenka. This will make computational and experimental studies more and more important in the future.
Further information:
The work was supported by the German Research Foundation (DFG) as part of the International Graduate School of Science and Engineering of the Technical University of Munich (TUM), the Czech Republic's Ministry for Youth, Education and Sports and the Central European Institute of Technology (CEITEC) in Brno (Czech Republic).
Prof. Dr. Roland A. Fischer
Director of the Catalysis Research Center (CRC)
Chair for Inorganic and Organometallic Chemistry
Technical University of Munich (TUM)
Ernst-Otto-Fischer-Straße 1, 85748 Garching,
Tel.: +” 89 289 13080 – E-mail: roland.fischer@tum.de
Web: https://www.department.ch.tum.de/amc/home/
Prof. Dr. Aliaksandr S. Bandarenka
Physics of Energy Conversion and Storage
Technical University of Munich (TUM)
James-Franck-Straße 1, 85748 Garching
Tel.: +” 89 289 12531 – E-mail: bandarenka@ph.tum.de
Web: https://www.groups.ph.tum.de/energy/ecs/
Optimizing the Size of Platinum Nanoparticles for Enhanced Mass Activity in the Electrochemical Oxygen Reduction Reaction
Batyr Garlyyev, Kathrin Kratzl, Marlon Rück, Jan Michalicka, Johannes Fichtner, Jan M. Macak, Tim Kratky, Sebastian Günther, Mirza Cokoja, Aliaksandr S. Bandarenka, Alessio Gagliardi and Roland A. Fischer
Angewandte Chemie May 3, 2019 – DOI: 10.1002/anie.20190492
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201904492
https://www.tum.de/nc/en/about-tum/news/press-releases/details/35554/ Link to the press release
Media Contact
All latest news from the category: Interdisciplinary Research
News and developments from the field of interdisciplinary research.
Among other topics, you can find stimulating reports and articles related to microsystems, emotions research, futures research and stratospheric research.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…