Solar-to-fuel system recycles CO2 to make ethanol and ethylene
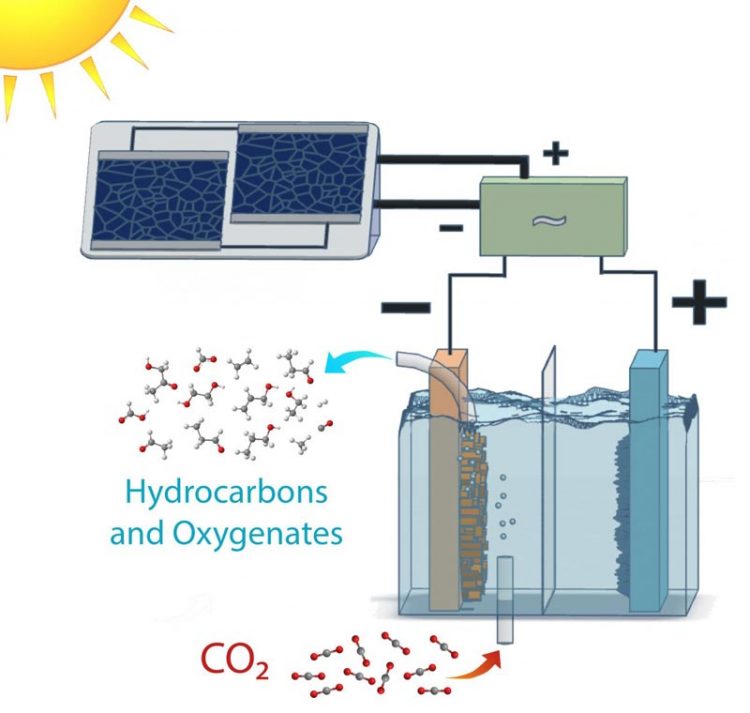
This is a schematic of a solar-powered electrolysis cell which converts carbon dioxide into hydrocarbon and oxygenate products with an efficiency far higher than natural photosynthesis. Power-matching electronics allow the system to operate over a range of sun conditions. Credit: Clarissa Towle/Berkeley Lab
Scientists at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have harnessed the power of photosynthesis to convert carbon dioxide into fuels and alcohols at efficiencies far greater than plants. The achievement marks a significant milestone in the effort to move toward sustainable sources of fuel.
Many systems have successfully reduced carbon dioxide to chemical and fuel precursors, such as carbon monoxide or a mix of carbon monoxide and hydrogen known as syngas. This new work, described in a study published in the journal Energy and Environmental Science, is the first to successfully demonstrate the approach of going from carbon dioxide directly to target products, namely ethanol and ethylene, at energy conversion efficiencies rivaling natural counterparts.
The researchers did this by optimizing each component of a photovoltaic-electrochemical system to reduce voltage loss, and creating new materials when existing ones did not suffice.
“This is an exciting development,” said study principal investigator Joel Ager, a Berkeley Lab scientist with joint appointments in the Materials Sciences and the Chemical Sciences divisions. “As rising atmospheric CO2 levels change Earth's climate, the need to develop sustainable sources of power has become increasingly urgent. Our work here shows that we have a plausible path to making fuels directly from sunlight.”
That sun-to-fuel path is among the key goals of the Joint Center for Artificial Photosynthesis (JCAP), a DOE Energy Innovation Hub established in 2010 to advance solar fuel research. The study was conducted at JCAP's Berkeley Lab campus.
The initial focus of JCAP research was tackling the efficient splitting of water in the photosynthesis process. Having largely achieved that task using several types of devices, JCAP scientists doing solar-driven carbon dioxide reduction began setting their sights on achieving efficiencies similar to those demonstrated for water splitting, considered by many to be the next big challenge in artificial photosynthesis.
Another research group at Berkeley Lab is tackling this challenge by focusing on a specific component in a photovoltaic-electrochemical system. In a study published today, they describe a new catalyst that can achieve carbon dioxide to multicarbon conversion using record-low inputs of energy.
Not just for noon
For this JCAP study, researchers engineered a complete system to work at different times of day, not just at a light energy level of 1-sun illumination, which is equivalent to the peak of brightness at high noon on a sunny day. They varied the brightness of the light source to show that the system remained efficient even in low light conditions.
When the researchers coupled the electrodes to silicon photovoltaic cells, they achieved solar conversion efficiencies of 3 to 4 percent for 0.35 to 1-sun illumination. Changing the configuration to a high-performance, tandem solar cell connected in tandem yielded a conversion efficiency to hydrocarbons and oxygenates exceeding 5 percent at 1-sun illumination.
“We did a little dance in the lab when we reached 5 percent,” said Ager, who also holds an appointment as an adjunct professor at UC Berkeley's Materials Science and Engineering Department.
Among the new components developed by the researchers are a copper-silver nanocoral cathode, which reduces the carbon dioxide to hydrocarbons and oxygenates, and an iridium oxide nanotube anode, which oxidizes the water and creates oxygen.
“The nice feature of the nanocoral is that, like plants, it can make the target products over a wide range of conditions, and it is very stable,” said Ager.
The researchers characterized the materials at the National Center for Electron Microscopy at the Molecular Foundry, a DOE Office of Science User Facility at Berkeley Lab. The results helped them understand how the metals functioned in the bimetallic cathode. Specifically, they learned that silver aids in the reduction of carbon dioxide to carbon monoxide, while the copper picks up from there to reduce carbon monoxide further to hydrocarbons and alcohols.
Seeking better, low-energy breakups
Because carbon dioxide is a stubbornly stable molecule, breaking it up typically involves a significant input of energy.
“Reducing CO2 to a hydrocarbon end product like ethanol or ethylene can take up to 5 volts, start to finish,” said study lead author Gurudayal, postdoctoral fellow at Berkeley Lab. “Our system reduced that by half while maintaining the selectivity of products.”
Notably, the electrodes operated well in water, a neutral pH environment.
“Research groups working on anodes mostly do so using alkaline conditions since anodes typically require a high pH environment, which is not ideal for the solubility of CO2,” said Gurudayal. “It is very difficult to find an anode that works in neutral conditions.”
The researchers customized the anode by growing the iridium oxide nanotubes on a zinc oxide surface to create a more uniform surface area to better support chemical reactions.
“By working through each step so carefully, these researchers demonstrated a level of performance and efficiency that people did not think was possible at this point,” said Berkeley Lab chemist Frances Houle, JCAP deputy director for Science and Research Integration, who was not part of the study. “This is a big step forward in the design of devices for efficient CO2 reduction and testing of new materials, and it provides a clear framework for the future advancement of fully integrated solar-driven CO2-reduction devices.”
###
Other co-authors on the study include James Bullock, a Berkeley Lab postdoctoral researcher in materials sciences, who was instrumental in engineering the system's photovoltaic and electrolysis cell pairing. Bullock works in the lab of study co-author Ali Javey, Berkeley Lab senior faculty scientist and a UC Berkeley professor of electrical engineering and computer sciences.
This work is supported by the DOE Office of Science.
Lawrence Berkeley National Laboratory addresses the world's most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab's scientific expertise has been recognized with 13 Nobel Prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy's Office of Science. For more, visit http://www.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media Contact
All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…