New electrolyte stops rapid performance decline of next-generation lithium battery
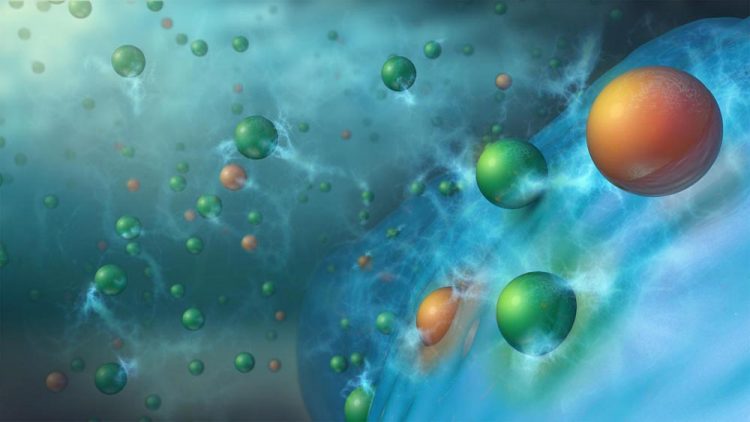
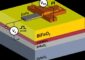
Charging results in doubly or triply charged metal cations, such as Mg2+ (orange spheres), along with singly charged lithium ions (green spheres) being co-inserted from the electrolyte into the silicon (blue spheres) anode material. This process stabilizes the anode, enabling long term cycling of lithium-ion batteries. Credit: Argonne National Laboratory
The lithium-ion battery is ubiquitous. Because of its versatility, this battery can be tailored to powering cell phones, laptops, power tools or electric vehicles. It is now the source of a multibillion-dollar enterprise annually that continues to grow each year.
Researchers at the U.S. Department of Energy's (DOE) Argonne National Laboratory have developed a new electrolyte mixture and a simple additive that could have a place in the next generation of lithium-ion batteries.
“Based on these test results, we have every reason to believe that, if silicon anodes ever replace graphite or constitute the anode in more than a few percent concentration, this invention will be part of it and could have far reaching impact.” — Baris Key, Argonne chemist
For many decades, scientists have been vigorously hunting for new electrode materials and electrolytes that can produce a new generation of lithium-ion batteries offering much greater energy storage while lasting longer, costing less and being safer. This new generation will likely make electric vehicles more widespread and accelerate the electric grid's expansion into renewable energy through cheaper and more reliable energy storage.
For scientists developing advanced lithium-ion batteries, the silicon anode has been the preeminent candidate to replace the current graphite anode. Silicon has a significant theoretical energy storage capacity advantage over graphite, being able to store almost ten times the lithium as does graphite. Increasing silicon's attractiveness commercially is its low cost. It is the second most abundant material in the Earth's crust, and its prevalence in computing and telecommunications hardware means there exist substantial processing technologies.
“But a stumbling block has remained,” noted Jack Vaughey, a senior chemist in Argonne's Chemical Sciences and Engineering (CSE) division. “On cycling, a silicon-based anode in a lithium-ion cell becomes very reactive with the electrolyte, and this process degrades the cell over time, causing a shortened cycle life.”
Lithium-ion battery electrolytes currently contain a solvent mixture, with a dissolved lithium salt and at least one, often more than three organic additives. Argonne scientists have developed a unique electrolyte additive strategy — a small amount of a second salt containing any one of several doubly or triply charged metal cations (Mg2+, Ca2+, Zn2+, or Al3+). These enhanced electrolyte mixtures, collectively named “MESA” (which stands for mixed-salt electrolytes for silicon anodes), give silicon anodes increased surface and bulk stabilities, improving long-term cycling and calendar life.
“We have thoroughly tested MESA formulations with full cells fabricated with standard commercially relevant electrodes,” said Baris Key, a chemist in the CSE division. “The new chemistry is simple, scalable and fully compatible with existing battery technology.”
“In this project, we greatly benefited from Argonne's Cell Analysis, Modeling and Prototyping (CAMP) facility,” added Vaughey. “It was there that we tested our MESA formulations.”
The Argonne researchers also investigated how the MESA-containing electrolytes work. During charging, the metal cation additions in electrolyte solution migrate into the silicon-based anode along with the lithium ions to form lithium-metal-silicon phases, which are more stable than lithium-silicon. This new cell chemistry greatly reduces the detrimental side reactions between the silicon anode and electrolyte that had plagued cells with the traditional electrolyte. Of the four metal salts tested in cells, the added electrolyte salts with either magnesium (Mg2+) or calcium (Ca2+) cations proved to work the best over hundreds of charge-discharge cycles. The energy densities obtained with these cells surpassed those for comparable cells having graphite chemistry by up to 50%.
“Based on these test results”, said Key, “We have every reason to believe that, if silicon anodes ever replace graphite or constitute the anode in more than a few percent concentration, this invention will be part of it and could have far reaching impact.”
###
The research was funded by the Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office.
A paper based on the study, “Using mixed salt electrolytes to stabilize silicon anodes for lithium-ion batteries via in situ formation of Li-M-Si Ternaries (M=Mg, Zn, Al, Ca),” appeared in ACS Applied Materials & Interfaces. Besides Vaughey and Key, other Argonne authors include Binghong Han, Chen Liao, Fulya Dogan, Stephen E. Trask and Saul H. Lapidus.
In performing some of this research, the scientists utilized two U.S. Department of Energy (DOE) Office of Science User Facilities located at Argonne: the Advanced Photon Source (APS) and the Center for Nanoscale Materials (CNM). They conducted high-energy X-ray diffraction measurements at beamline 11-BM of the APS, and scanning electron microscopy and transmission electron microscopy at the CNM.
About Argonne's Center for Nanoscale Materials
The Center for Nanoscale Materials is one of the five DOE Nanoscale Science Research Centers, premier national user facilities for interdisciplinary research at the nanoscale supported by the DOE Office of Science. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge, Sandia and Los Alamos National Laboratories. For more information about the DOE NSRCs, please visit https:/
About the Advanced Photon Source
This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https:/
Media Contact
All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…