The Slipperiness of Ice Explained
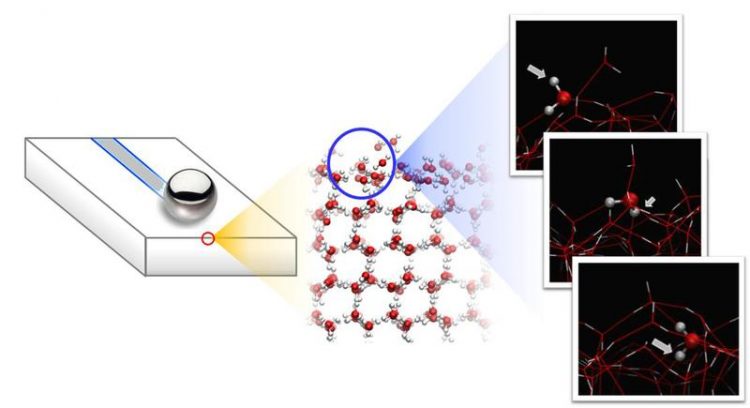
In the experiments, a steel ball slides over the ice surface which consists of rapidly tumbling mobile water molecules that are only loosely bounded to the underlying ice. © Nagata/MPI-P
Winter sports such as skiing, speed skating, figure skating, and curling require the slippery surfaces of ice and snow. While the fact that the ice surface is slippery is widely acknowledged, it is far from being completely understood.
In 1886 John Joly, an Irish physicist, offered the first scientific explanation for low friction on ice; when an object – i.e. an ice skate – touches the ice surface the local contact pressure is so high that the ice melts thereby creating a liquid water layer that lubricates the sliding.
The current consensus is that although liquid water at the ice surface does reduce sliding friction on ice, this liquid water is not melted by pressure but by frictional heat produced during sliding.
A team of researchers led by brothers Prof. Daniel Bonn from the University of Amsterdam and Prof. Mischa Bonn from MPI-P, have now demonstrated that friction on ice is more complex than so far assumed.
Through macroscopic friction experiments at temperatures ranging from 0 °C to -100 °C the researchers show that – surprisingly – the ice surface transforms from an extremely slippery surface at typical winter sports temperatures, to a surface with high friction at -100 °C.
To investigate the origin of this temperature-dependent slipperiness, the researchers performed spectroscopic measurements of the state of water molecules at the surface, and compared these with molecular dynamics (MD) simulations.
This combination of experiment and theory reveals that two types of water molecules exist at the ice surface: water molecules that are stuck to the underlying ice (bound by three hydrogen bonds) and mobile water molecules that are bound by only two hydrogen bonds. These mobile water molecules continuously roll over the ice – like tiny spheres – powered by thermal vibrations.
As the temperature increases, the two species of surface molecules are interconverted: the number of mobile water molecules is increased at the expense of water molecules that are fixed to the ice surface. Remarkably, this temperature driven change in the mobility of the topmost water molecules at the ice surface perfectly matches the temperature-dependence of the measured friction force: the larger the mobility at the surface, the lower the friction and vice versa.
The researchers therefore conclude that – rather than a thin layer of liquid water on the ice – the high mobility of the surface water molecules is responsible for the slipperiness of ice.
Although the surface mobility continues to increase all the way up to 0 °C, this is not the ideal temperature for sliding on ice. The experiments show that the friction is in fact minimal at -7 °C; the exact same temperature is imposed at speed skating rinks.
The researchers show that at temperatures between -7 °C and 0 °C, sliding is more difficult because the ice becomes softer, causing the sliding object to dig deeper into the ice.
The results are published in the Journal of Physical Chemistry Letters.
Contact:
Prof. Daniel Bonn
Postbus 94485
1090 GL Amsterdam
eMail: d.bonn@uva.nl
phone: +31 (0)205255887
Prof. Mischa Bonn
Max Planck Institute for Polymer Research
Ackermannweg 10
D-55128 Mainz
eMail: bonn@mpip-mainz.mpg.de
phone: +49 (0)6131 379 161
Link to publication:
https://pubs.acs.org/doi/10.1021/acs.jpclett.8b01188
Link to Max Planck Institute for Polymer Research:
https://www.mpip-mainz.mpg.de/5334049/PM2018-12
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















