New Surface Can Find Different Twists on a Molecular Theme
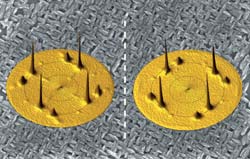
The copper-colored images in the above figure are x-ray pole figures of copper-oxide (CuO) films that researchers at the University of Missouri-Rolla have deposited onto a gold surface. The grey background is a scanning electron microscope image of one of the CuO films. The researchers created the pole figures—which represent data, not images of molecules—using an advanced measuring instrument called an x-ray diffractometer. Scientists use x-ray pole figures to determine the atomic structure and orientation of crystalline materials. As with a person’s right and left hands, the CuO films (and their pole figures) cannot be superimposed on one another. This concept is called chirality, and is a characteristic of many biologically-important molecules. The CuO films have been shown to distinguish between the left- and right-handed versions of molecules, an important trait researchers can use to create new chemical sensors and catalysts. <br>Credit: Jay Switzer and Eric Bohannan, University of Missouri-Rolla; National Science Foundation
Researchers have created a new process to produce materials that can sift through similar, molecular brethren and latch onto chemicals that differ from each other in only their mirrored images.
If it proves effective in large-scale experiments, the stable, relatively simple catalyst could impact the $100 billion pharmaceutical industry by helping sort biologically potent chemicals from related, yet less useful or even toxic, compatriots.
Jay Switzer and colleagues at the University of Missouri at Rolla announce their discovery in the October 2, 2003, issue of the journal Nature. The research was funded primarily by the Division of Chemistry and the Division of Material Research at the National Science Foundation (NSF), the independent federal agency that supports research and education across all fields of science and engineering.
The new material, a thin film of copper oxide on a layer of gold, has an inherent “handedness.” Just as human hands come in a left and right-handed variety, so do some molecules. “It’s just like the difference between shaking hands between a right- or left-handed person,” said NSF program officer and chemistry expert Mike Clarke. “The handclasp is much easier if it’s right-right or left-left, and harder if it’s right-left or left-right.” The handedness property, called “chirality,” is fundamentally related to how the molecule reacts with other substances.
Approximately one-third of all drugs are chiral, says Switzer, and the top-10 list for these products includes such familiar brand names as Lipitor, Zocor, Paxil, Zoloft, and Nexium-all of which yield sales of over $1 billion a year.
Currently, most industries make bulk quantities of chiral molecules by mixing handed molecules and other chemicals in a solution. “The ’hands’ gather atoms, assembling chiral, molecular ’gloves,’” said chemist Katherine Covert, one of the NSF program officers who oversees funding of Switzer’s research.
“But, separating the useful ’gloves’ from the chemicals that assembled them can be a difficult process,” she added.
Switzer and his colleagues have created a material that serves as a bank of “hands,” a solid structure on which reactions can occur and from which researchers can more easily separate desirable chemicals.
To do this, the group used tartrate, a common substance that often crystallizes on the bottom of wine corks. In 1848, when Louis Pasteur was 26, he used tweezers to separate right- and left-handed forms of sodium ammonium tartrate under a microscope.
“The crystals have a different shape,” said Switzer. “When Pasteur dissolved the crystals in water, one set rotated polarized light to the right, and the other form rotated it to the left. This experiment is usually cited as the discovery of chirality in molecules,” he added.
Tartrate was therefore the first molecule ever isolated in right handed and left-handed forms, which chemists now refer to as the R (from the Latin rectus) and S (from the Latin sinister) forms.
“We’ve made a material that is entirely chiral, not just the surface,” said Switzer. “In earlier experiments, you would modify a surface with a chiral modifying agent, and if the agent washes off, the surface is no longer effective. In our new research, the film itself is chiral-the effectiveness remains even after many chemical reactions,” he added.
To create the new catalyst, the researchers immersed gold in a liquid containing copper and the handed chemical tartrate, while applying an electric current to the system. The electricity caused copper oxide to bond to the gold. The technique is much like the simple electroplating used to produce the shiny chrome on automobiles.
The gold atoms were highly organized in a crystal structure, but the structure was symmetrical and did not affect the final handedness of the material. Instead, the chiral structure of the tartrate molecules caused the copper oxide to bind to the gold layer-by-layer and in an oriented fashion, creating a handed film.
Once the copper oxide film forms, the copper and oxygen atoms are tightly attached and exist as a single material. In initial tests, the researchers have shown the material can differentiate between chiral tartrate without breaking down or being permanently altered. Pending tests will apply the same technique to amino acids and sugars, such as glucose.
Now, Switzer and his colleagues hope to use their process to create new catalysts and separate different chemicals. In addition to numerous industry uses, the researchers hope similar catalysts may eventually be modified to create sensors for security applications.
Switzer is supported by NSF through both the Division of Chemistry and the Division of Materials Research.
Trademarks for prescription drug names that appear in this press release – Lipitor, Zocor, Paxil, Zoloft, and Nexium – are the trademarks of their respective owners.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















