Prediction of titanic nitride proved unsinkable
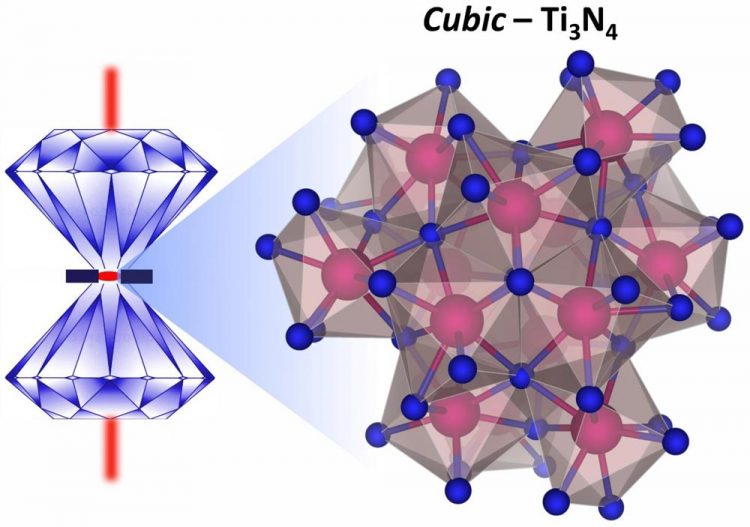
An illustration of how cubic titanium nitride with a three-to-four ratio can be synthesized under extreme pressures and temperatures in a laser-heated diamond anvil cell. Credit: Image is courtesy of Venkata Bhadram
A team of experimental and computational scientists led by Carnegie's Tim Strobel and Venkata Bhadram have synthesized a long sought-after form of titanium nitride, Ti3N4, which has promising mechanical and optoelectronic properties.
Standard titanium nitride (TiN), with a one-to-one ratio of titanium and nitrogen, exhibits a crystal structure resembling that of table salt–sodium chloride, or NaCl. It is a metal with abrasive properties and thus used for tool coatings and manufacturing of electrodes.
Titanium nitride with a three-to-four ratio of titanium and nitrogen, called titanic nitride, has remained elusive, despite previous theoretical predictions of its existence and the fact that nitrides with this ratio have been identified for the other members of titanium's group on the period table, including zirconium.
Strobel and Bhadram's team–Carnegie's Hanyu Liu , and Rostislav Hrubiak, as well as Vitali B. Prakapenka of the University of Chicago, Enshi Xu and Tianshu Li of George Washington University, and Stephan Lany of the National Renewable Energy Laboratory –undertook the challenge. Their work is published and highlighted as an Editor's Suggestion in Physical Review Materials.
They created Ti3N4 in a cubic crystalline phase using a laser-heated diamond anvil cell, which was brought to about 740,000 times normal atmospheric pressure (74 gigapascals) and about 2,200 degrees Celsius (2,500 kelvin).
Advanced x-ray and spectroscopic tools confirmed the crystalline structure the team had created under these conditions, and theoretical model-based calculations allowed them to predict the thermodynamic nature and physical properties of Ti3N4.
Table-salt-like TiN is metallic, which means it can conduct a flow of electrons that makes up a current. But cubic Ti3N4 is a semiconductor, which means that it can have its electrical conductivity turned on and off. This possibility is tremendously useful in electronic devices. Titanium-based semiconductors are particularly popular as catalysts for solar water-splitting reactions to produce hydrogen, a clean renewable-energy source.
This ability to switch conductivity on and off is possible because some of a semiconductor's electrons can move from lower-energy insulating states to higher-energy conducting states when subjected to an input of energy. The energy required to initiate this leap is called a band gap. The band gap for cubic Ti3N4 is larger than expected based on previous model predictions. Furthermore, like metallic TiN, Ti3N4 is expected to exhibit excellent mechanical and wear resistance properties.
“To our knowledge this is the first experimental report on semiconducting titanium nitride” said lead author Bhadram. “We believe that this work will stimulate further experimental and theoretical efforts to design new ways to scale up the synthesis of Ti3N4 at ambient pressure.”
###
This work was supported by Energy Frontier Research in Extreme Environments (EFree) Center, an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy, Office of Science.
Portions of this work were performed at GeoSoilEnviroCARS and HPCAT, Advanced Photon Source, Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation-Earth Sciences and Department of Energy-GeoSciences.
The Carnegie Institution for Science (carnegiescience.edu) is a private, nonprofit organization headquartered in Washington, D.C., with six research departments throughout the U.S. Since its founding in 1902, the Carnegie Institution has been a pioneering force in basic scientific research. Carnegie scientists are leaders in plant biology, developmental biology, astronomy, materials science, global ecology, and Earth and planetary science.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…

Rocks with the oldest evidence yet of Earth’s magnetic field
The 3.7 billion-year-old rocks may extend the magnetic field’s age by 200 million years. Geologists at MIT and Oxford University have uncovered ancient rocks in Greenland that bear the oldest…

Decisive breakthrough for battery production
Storing and utilising energy with innovative sulphur-based cathodes. HU research team develops foundations for sustainable battery technology Electric vehicles and portable electronic devices such as laptops and mobile phones are…





















