Melting solid below the freezing point
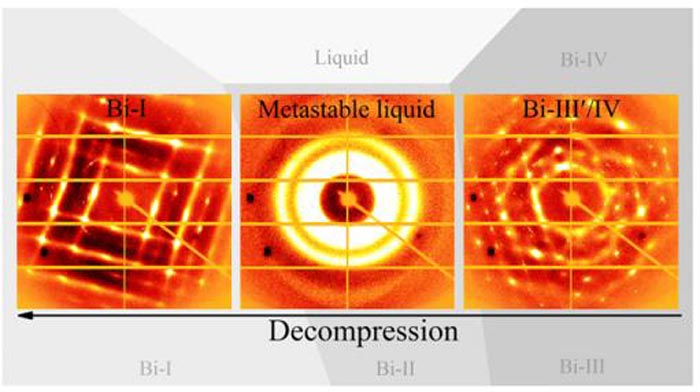
When a crystal structure of bismuth (right) is decompressed from 32,000 atmospheres (3.2 GPa) to 12,000 atmospheres (1.2 GPa) it melts into a liquid at about 23,000 atmospheres (2.3 GPa) (middle). It then recrystallizes at 12,000 atmospheres (left). The so-called metastable liquid produced by this decompression occurs in a pressure-temperature range similar to where the supercooled bismuth is produced. Supercooled liquids are cooled below the freezing point without turning into a solid or a crystal. Credit: Chuanlong Lin and Guoyin Shen, Carnegie Institution
The results, reported in the January 23, 2017, issue of Nature Communications, could be important for developing new materials and for understanding the dynamics of planetary interiors, such as earthquakes, because a metastable liquid could act as a lubricant strongly affecting the dynamics of the Earth's interior.
“Phase transitions come in two basic 'flavors,'” explained Carnegie co-author Guoyin Shen, director of the High-Pressure Collaborative Access Team at the Advanced Photon Source*. “In one type, the chemical bonds do not break as the material goes from one phase to another. But they do alter in orientation and length in an orderly manner.
The other, called reconstructive phase transition, is more chaotic, but the most prevalent in nature and the focus of this study. In these transitions, parts of the chemical bonds are broken and the structure changes significantly when it enters a new phase.”
Pressure can be used to change the phase of a material in addition to heating and cooling. The scientists put a form of crystalline bismuth in a pressure-inducing diamond anvil cell, and subjected it to pressures and decompression ranging from 32,000 times atmospheric pressure (3.2 GPa) to 12,000 atmospheres (1.2 GPa) at a temperature of 420° F (489 K). Under decompression only, at about 23,000 atmospheres, bismuth melts into a liquid. Then at 12,000 atmospheres it recrystallizes.
“The richness in crystalline structure of bismuth is particularly useful for witnessing changes in the structure of a material,” remarked lead author Chuanlong Lin.
The researchers imaged the changes using a technique called X-ray diffraction, which uses much higher energy X-rays than those we use for medical imaging and can therefore discern structure at the atomic level. They conducted five different compression/decompression rounds of experiments.
“The bismuth displayed a metastable liquid in the process of solid-solid phase transitions under decompression at about 23,000 to 15,000 atmospheres,” Lin said.
The scientists also found that the metastable state can endure for hours below the melting point under static conditions. Interestingly, the metastable liquid produced by decompression occurred in a pressure-temperature range that is similar to where supercooled bismuth is produced.
“Because reconstructive phase transitions are the most fundamental type, this research provides a brand new way for understanding how different materials change,” Shen said. “It's possible that other materials could display a similar metastable liquid when they undergo reconstructive transitions and that this phenomenon is more prevalent than we thought. The results will no doubt lead to countless surprises in both materials science and planetary science in the coming years.”
###
*Authors on the paper are from Carnegie's Geophysical Laboratory, High-Pressure Collaborative Access Team at Argonne National Laboratory: Chuanlong Lin, Jesse Smith, Stanislav Sinogeikin, Yoshio Kono, Changyong Park, Curtis Kenney-Benson, and Guoyin Shen. The work was performed at the Carnegie Institution for Science and Argonne National Laboratory, and is supported by the DOE/BES X-ray Scattering Program. HPCAT operation is supported by DOE-NNSA. The Advanced Photon Source is a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The Carnegie Institution for Science (carnegiescience.edu) is a private, nonprofit organization headquartered in Washington, D.C., with six research departments throughout the U.S. Since its founding in 1902, the Carnegie Institution has been a pioneering force in basic scientific research. Carnegie scientists are leaders in plant biology, developmental biology, astronomy, materials science, global ecology, and Earth and planetary science.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















