Mapping nanoscale chemical reactions inside batteries in 3-D
This is lithium iron phosphate. Credit: Jordi Cabana
“Knowing the precise locations of chemical reactions within individual nanoparticles that are participating in those reactions helps us to identify how a battery operates and uncover how the battery might be optimized to make it work even better,” said Jordi Cabana, associate professor of chemistry at UIC and co-corresponding author on the paper.
As a battery charges and discharges, its electrodes — the materials where the reactions that produce energy take place — are alternately oxidized and reduced. The chemical pathways by which these reactions take place help determine how quickly a battery becomes depleted.
Tools available to study these reactions can only provide information on the average composition of electrodes at any given point in time. For example, they can let a researcher know what percentage of the electrode has become permanently oxidized.
But these tools cannot provide information on the location of oxidized portions in the electrode. Because of these limitations, it is not possible to tell if reactions are confined to a certain area of the electrode, such as the surface of the material, or if reactions are taking place uniformly throughout the electrode.
“Being able to tell if there is a tendency for a reaction to take place in a specific part of the electrode, and better yet, the location of reactions within individual nanoparticles in the electrode, would be extremely useful because then you could understand how those localized reactions correlate with the behavior of the battery, such as its charging time or the number of recharge cycles it can undergo efficiently,” Cabana said.
The new technique, called X-ray ptychographic tomography, came about through a partnership between chemists at UIC and scientists at the Advanced Light Source, at Lawrence Berkeley National Laboratory in California. Advanced Light Source scientists developed the instrumentation and measurement algorithms, which were used to help answer fundamental questions about battery materials and behavior identified by the UIC team.
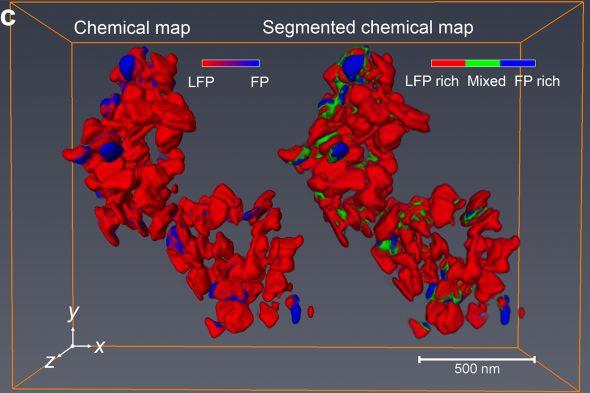
Together, the two teams used the tomographic technique to look at tens of nanoparticles of lithium-iron phosphate recovered from a battery electrode that had been partially charged. The researchers used a coherent, nanoscale beam of X-rays generated by the high-flux synchrotron accelerator at the Advanced Light Source to interrogate each nanoparticle. The pattern of absorption of the beam by the material gave the researchers information about the oxidation state of iron in the nanoparticles in the X-ray beam.
Because they were able to move the beam just a few nanometers over and run their interrogation again, the team could reconstruct chemical maps of the nanoparticles with a resolution of about 11 nanometers. By rotating the material in space, they could create a three-dimensional tomographic reconstruction of the oxidation states of each nanoparticle. In other words, they could tell the extent to which an individual nanoparticle of lithium iron phosphate had reacted.
“Using our new technique, we could not only see that individual nanoparticles showed different extents of reaction at a given time, but also how the reaction worked its way through the interior of each nanoparticle,” Cabana said.
###
The UIC chemists are members of the NorthEast Center for Chemical Energy Storage, an Energy Frontier Research Center funded by the Department of Energy to investigate how Li-ion batteries work so that better, longer-lasting and lighter devices can be designed.
David Shapiro of Lawrence Berkeley National Laboratories is the co-corresponding author on the paper. Young-Sang Yu, Maryam Farmand, Tolek Tyliszczak, Rich Celestre, Peter Denes, A. L. David Kilcoyne, Stefano Marchesini, Tony Warwick, John Joseph, Harinarayan Krishnan, Costa Leite and Howard Padmore of Lawrence Berkeley National Laboratory; Chunjoong Kim of the University of Illinois at Chicago; Yijin Liu of SLAC National Accelerator Laboratory, Menlo Park, California; Clare Grey, Fiona Strobridge of NECCES at the University of Cambridge; and Filipe Maia of Uppsala University, are co-authors on the paper.
This research was supported by the NorthEast Center for Chemical Energy Storage, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DESC0012583, a grant from the National Research Lab (NRF- 2015R1A2A1A01006192), a program of the National Research Foundation of Korea, by the Center for Applied Mathematics for Energy Research Applications, a partnership between Basic Energy Sciences and Advanced Scientific Computing Research at the U.S Department of Energy.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















