Lawrence Livermore scientists discover new materials to capture methane
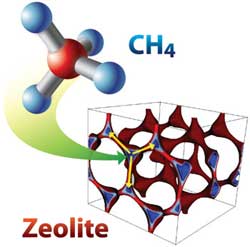
Methane capture in zeolite SBN. Blue represents adsorption sites, which are optimal for methane (CH4) uptake. Each site is connected to three other sites (yellow arrow) at optimal interaction distance. <br>
Methane is a substantial driver of global climate change, contributing 30 percent of current net climate warming. Concern over methane is mounting, due to leaks associated with rapidly expanding unconventional oil and gas extraction, and the potential for large-scale release of methane from the Arctic as ice cover continues to melt and decayed material releases methane to the atmosphere. At the same time, methane is a growing source of energy, and aggressive methane mitigation is key to avoiding dangerous levels of global warming.
The research team, made up of Amitesh Maiti, Roger Aines and Josh Stolaroff of LLNL and Professor Berend Smit, researchers Jihan Kim and Li-Chiang Lin at UC Berkeley and Lawrence Berkeley National Lab, performed systematic computer simulation studies on the effectiveness of methane capture using two different materials – liquid solvents and nanoporous zeolites (porous materials commonly used as commercial adsorbents).
While the liquid solvents were not effective for methane capture, a handful of zeolites had sufficient methane sorption to be technologically promising. The research appears in the April 16 edition of the journal, Nature Communications.
Unlike carbon dioxide, the largest emitted greenhouse gas, which can be captured both physically and chemically in a variety of solvents and porous solids, methane is completely non-polar and interacts very weakly with most materials.
“Methane capture poses a challenge that can only be addressed through extensive material screening and ingenious molecular-level designs,” Maiti said.
Methane is far more potent as a greenhouse gas than CO2. Researchers have found that the release of as little as 1 percent of methane from the Arctic alone could have a warming effect approaching that being produced by all of the CO2 that has been pumped into the atmosphere by human activity since the start of the Industrial Revolution.
Methane is emitted at a wide range of concentrations from a variety of sources, including natural gas systems, livestock, landfills, coal mining, manure management, wastewater treatment, rice cultivation and a few combustion processes.
The team's research focused on two different applications — concentrating a medium-purity methane stream to a high-purity range (greater than 90 percent), as involved in purifying a low-quality natural gas; and concentrating a dilute stream (about 1 percent or lower) to the medium-purity range (greater than 5 percent), above methane's flammability limit in air.
Through an extensive study, the team found that none of the common solvents (including ionic liquids) appears to possess enough affinity toward methane to be of practical use. However, a systematic screening of around 100,000 zeolite structures uncovered a few nanoporous candidates that appear technologically promising.
Zeolites are unique structures that can be used for many different types of gas separations and storage applications because of their diverse topology from various networks of the framework atoms. In the team's simulations, one specific zeolite, dubbed SBN, captured enough medium source methane to turn it to high purity methane, which in turn could be used to generate efficient electricity.
“We used free-energy profiling and geometric analysis in these candidate zeolites to understand how the distribution and connectivity of pore structures and binding sites can lead to enhanced sorption of methane while being competitive with CO2 sorption at the same time,” Maiti said.
Other zeolites, named ZON and FER, were able to concentrate dilute methane streams into moderate concentrations that could be used to treat coal-mine ventilation air.
The work at LLNL was funded by the Advanced Research Projects Agency-Energy (ARPA-E).
More Information
New materials for methane capture from dilute and medium-concentration sources
Nature Communications, April 16, 2013
A new method to cleaner and more efficient CO2 capture
LLNL news release, July 22, 2009
Dissolving Molecules to Improve Their Performance
Science & Technology Review, June 2009
Hydrocarbons in the deep earth
LLNL news release, April 14, 2011
The Search for Methane in Earth's Mantle
Science & Technology Review, July/August 2005
Founded in 1952, Lawrence Livermore National Laboratory provides solutions to our nation's most important national security challenges through innovative science, engineering and technology. Lawrence Livermore National Laboratory is managed by Lawrence Livermore National Security, LLC for the U.S. Department of Energy's National Nuclear Security Administration.
Media Contact
More Information:
http://www.llnl.govAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















