'Diamonds from the sky' approach turns CO2 into valuable products
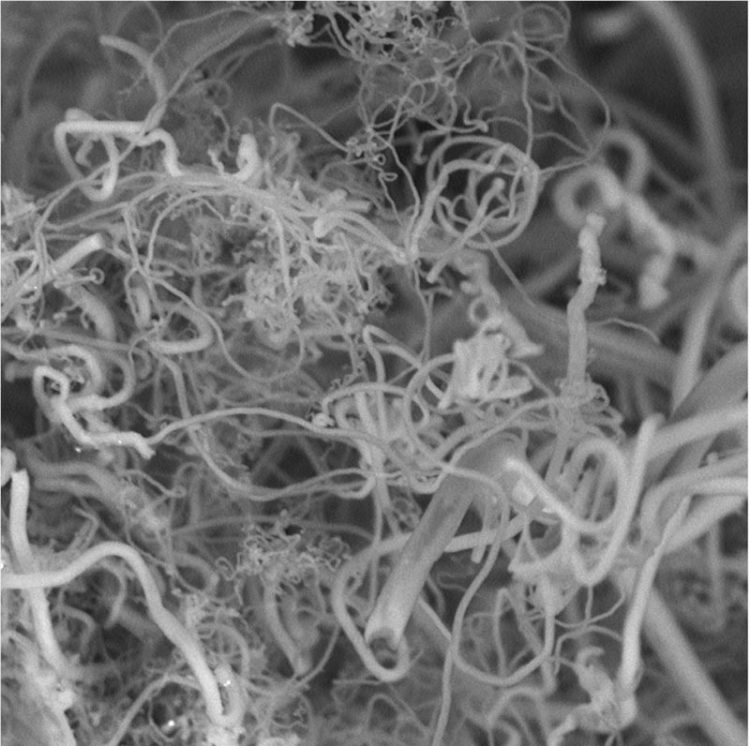
Researchers are generating carbon nanofibers (above) from CO2, removing a greenhouse gas from the air to make products. Credit: Stuart Licht, Ph.D.
The team will present brand-new research on this new CO2 capture and utilization technology at the 250th National Meeting & Exposition of the American Chemical Society (ACS). ACS is the world's largest scientific society. The national meeting, which takes place here through Thursday, features more than 9,000 presentations on a wide range of science topics.
“We have found a way to use atmospheric CO2 to produce high-yield carbon nanofibers,” says Stuart Licht, Ph.D., who leads a research team at George Washington University. “Such nanofibers are used to make strong carbon composites, such as those used in the Boeing Dreamliner, as well as in high-end sports equipment, wind turbine blades and a host of other products.”
Previously, the researchers had made fertilizer and cement without emitting CO2 , which they reported. Now, the team, which includes postdoctoral fellow Jiawen Ren, Ph.D., and graduate student Jessica Stuart, says their research could shift CO2 from a global-warming problem to a feed stock for the manufacture of in-demand carbon nanofibers.
Licht calls his approach “diamonds from the sky.” That refers to carbon being the material that diamonds are made of, and also hints at the high value of the products, such as the carbon nanofibers that can be made from atmospheric carbon and oxygen.
Because of its efficiency, this low-energy process can be run using only a few volts of electricity, sunlight and a whole lot of carbon dioxide. At its root, the system uses electrolytic syntheses to make the nanofibers. CO2 is broken down in a high-temperature electrolytic bath of molten carbonates at 1,380 degrees F (750 degrees C). Atmospheric air is added to an electrolytic cell. Once there, the CO2 dissolves when subjected to the heat and direct current through electrodes of nickel and steel. The carbon nanofibers build up on the steel electrode, where they can be removed, Licht says.
To power the syntheses, heat and electricity are produced through a hybrid and extremely efficient concentrating solar-energy system. The system focuses the sun's rays on a photovoltaic solar cell to generate electricity and on a second system to generate heat and thermal energy, which raises the temperature of the electrolytic cell.
Licht estimates electrical energy costs of this “solar thermal electrochemical process” to be around $1,000 per ton of carbon nanofiber product, which means the cost of running the system is hundreds of times less than the value of product output.
“We calculate that with a physical area less than 10 percent the size of the Sahara Desert, our process could remove enough CO2 to decrease atmospheric levels to those of the pre-industrial revolution within 10 years,” he says.
At this time, the system is experimental, and Licht's biggest challenge will be to ramp up the process and gain experience to make consistently sized nanofibers. “We are scaling up quickly,” he adds, “and soon should be in range of making tens of grams of nanofibers an hour.”
Licht explains that one advance the group has recently achieved is the ability to synthesize carbon fibers using even less energy than when the process was initially developed. “Carbon nanofiber growth can occur at less than 1 volt at 750 degrees C, which for example is much less than the 3-5 volts used in the 1,000 degree C industrial formation of aluminum,” he says.
###
A press conference on this topic will be held Wednesday, Aug. 19, at 9:30 a.m. Eastern time in the Boston Convention & Exhibition Center. Reporters may check-in at Room 153B in person, or watch live on YouTube http://bit.
The team's research has been funded primarily by the National Science Foundation.
The American Chemical Society is a nonprofit organization chartered by the U.S. Congress. With more than 158,000 members, ACS is the world's largest scientific society and a global leader in providing access to chemistry-related research through its multiple databases, peer-reviewed journals and scientific conferences. Its main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact newsroom@acs.org.
Note to journalists: Please report that this research is being presented at a meeting of the American Chemical Society.
Title
A new approach to carbon dioxide utilization: The carbon molten air battery
Abstract
As the levels of carbon dioxide (CO2 ) increase in the Earth's atmosphere, the effects on climate change become increasingly apparent. As the demand to reduce our dependence on fossils fuels and lower our carbon emissions increases, a transition to renewable energy sources is necessary. Cost effective large-scale electrical energy storage must be established for renewable energy to become a sustainable option for the future. We've previously shown that carbon dioxide can be captured directly from the air at solar efficiencies as high as 50%, and that carbon dioxide associated with cement formation and the production of other commodities can be electrochemically avoided in the STEP process.1-3
The carbon molten air battery, presented by our group in late 2013, is attractive due to its scalability, location flexibility, and construction from readily available resources, providing a battery that can be useful for large scale applications, such as the storage of renewable electricity.4
Uncommonly, the carbon molten air battery can utilize carbon dioxide directly from the air:
(1) charging: CO2(g) -> C(solid) + O2(g)
(2) discharging: C(solid) + O2(g) -> CO2(g)
More specifically, in a molten carbonate electrolyte containing added oxide, such as lithium carbonate with lithium oxide, the 4 electron charging reaction eq. 1 approaches 100% faradic efficiency and can be described as the following two equations:
(1a) O2-(dissolved) + CO2(g) -> CO32-(molten)
(1b) CO32-(molten) -> C(solid) + O2(g) + O2-(dissolved)
Thus, powered by carbon formed directly from the CO2 in our earth's atmosphere, the carbon molten air battery is a viable system to provide large-scale energy storage.
1) S. Licht, “Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach” Advanced Materials, 47, 5592 (2011).
2) S. Licht, H. Wu, C. Hettige, B. Wang, J. Lau, J. Asercion, J. Stuart “STEP Cement: Solar Thermal Electrochemical Production of CaO without CO2 emission,” Chemical Communications, 48, 6019 (2012).
3) S. Licht, B. Cui, B. Wang, F.-F. Li, J. Lau, S. Liu,”Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3,” Science, 345, 637 (2014).
4) S. Licht, B. Cui, J. Stuart, B. Wang, J. Lau,” “Molten Air Batteries – A new, highest energy class of rechargeable batteries, Energy & Environmental Science, 6, 3646 (2013).
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















