Crystallization frustration predicts metallic glass formation
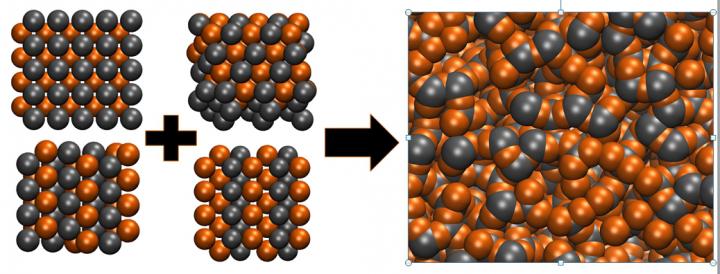
This is a visual representation of the difference between an organized, crystalline structure and an amorphous glass structure. Credit: Eric Perim Martins, Duke University
Researchers have discovered a way to predict which alloys will form metallic glasses. The research could pave the way for new strong, conductive materials.
Metallic glasses are sometimes formed when molten metal is cooled too fast for its atoms to arrange in a structured, crystalline order. The result is a material with numerous desirable properties. Because they are metals, metallic glasses have high hardness and toughness and good thermal conductivity. Because their structure is disorganized, they are easy to process and shape and difficult to corrode. Thanks to these characteristics, metallic glasses are used in a wide array of applications, including electrical applications, nuclear reactor engineering, medical industries, structural reinforcement and razor blades.
While metallic glass has been around for decades, scientists have no clue which combinations of elements will form them. The only way to come up with new metallic glasses to date has been to cook up new recipes in the laboratory with only a few rules of thumb for guidance and hope for the best — a costly endeavor in both time and money.
In a new study, however, researchers from Duke University, in collaboration with groups from Harvard University and Yale University, describe a method that can predict which binary alloys will form metallic glasses. Their technique involves computing and comparing the many pockets of different structures and energies that could be found within a solidified alloy.
The results were published August 2, 2016, in Nature Communications.
“When you get a lot of structures forming next to one another that are different but still have similar internal energies, you get a sort of frustration as the material tries to crystalize,” said Eric Perim, a postdoctoral researcher working in the laboratory of Stefano Curtarolo, professor of mechanical engineering and materials science and director of the Center for Materials Genomics at Duke. “The material can't decide which crystalline structure it wants to converge to, and a metallic glass emerges. What we created is basically a measure of that confusion.”
To determine the likelihood of an alloy forming a glass, Curtarolo, Perim and their colleagues broke its chemistry down into numerous sections, each containing only a handful of atoms. They then turned to a prototype database to simulate the hundreds of structures each section could potentially take.
Called the AFLOW library, the database stores information on atomic structures that are commonly observed in nature. Using these examples, the program computes what a novel combination of elements would look like with these structures. For example, the atomic structure of sodium chloride — better known as salt — may be used to build a potential structure for copper zirconium.
These simulations produce estimations of characteristics for hundreds of structural forms that a material could take. One characteristic, called an atomic environment, looks at the geometrical arrangement of an atom's closest neighbors. Another calculates the amount of energy stored in each of these atomic structures.
To determine the likelihood of an alloy forming a metallic glass, the program compares these two characteristics between the hundreds of different structures that could be found throughout the material. If groups of atoms near one another have similar energies, they want to form similar structures. But if the rapid cooling prevents this, a metallic glass emerges.
“The big advantage to our work is that it's high-throughput, because doing this experimentally is way too time-consuming,” said Cormac Toher, an assistant research professor in Curtarolo's laboratory. “You cannot check all compositions of all systems in the laboratory. That would literally take forever. The idea behind this is that we can screen a large number of materials in a couple of days and single out the most likely ones that should be checked out.”
The group then put their confusion-measuring program to the test to see if it could accurately predict metallic glasses that are already known. They were able to correctly identify 73 percent — a number they hope will improve as they continue to increase the structural information and simulations stored in their database.
Based on their initial work, they believe about one-sixth of the alloys in their system should make metallic glass. That's more than 250 potential materials, of which only about a couple dozen have been discovered.
“If you go to Venice you'll see people blowing bottles of glass,” said Curtarolo. “You can do that with metallic glasses as well. You can make lightweight, very durable objects without any seams. But trying to scale these up is difficult. The larger the lump, the longer it takes its center to cool, and the more likely it is to form a normal crystalline structure. But there might be undiscovered chemical combinations that would be easier to work with, cost less, or have other, more desirable properties. We just have to figure out where to look for them.”
Besides refining their results for binary alloys, the researchers plan to extend their algorithm to alloys that contain three elements, as they are more likely to form glasses but are much more difficult and time-consuming to model. Their database, however, has only about one-tenth of the entries for these alloys as it does for binary alloys, so computer clusters around the world will first need to work for some time to come.
###
This research was supported by the National Science Foundation (DMR-1436151, DMR-1436268, DMR-1435820).
CITATION: “Spectral Descriptors for Bulk Metallic Glasses Based on the Thermodynamics of Competing Crystalline Phases.” Eric Perim, Dongwoo Lee, Yanhui Liu, Cormac Toher, Pan Gong, Yanglin Li, W. Neal Simmons, Ohad Levy, Joost J. Vlassak, Jan Schroers and Stefano Curtarolo. Nature Communications, Aug. 2, 2016. DOI: 10.1038/NCOMMS12315
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…