Copper clusters capture and convert carbon dioxide to make fuel
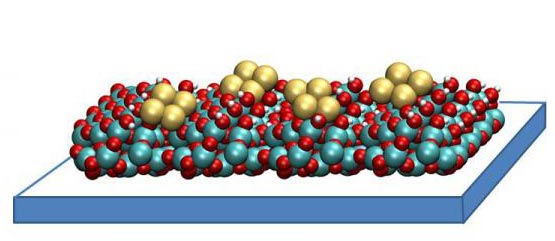
A copper tetramer catalyst created by researchers at Argonne National Laboratory may help capture and convert carbon dioxide in a way that ultimately saves energy. It consists of small clusters of four copper atoms each, supported on a thin film of aluminum oxide. These catalysts work by binding to carbon dioxide molecules, orienting them in a way that is ideal for chemical reactions. The structure of the copper tetramer is such that most of its binding sites are open, which means it can attach more strongly to carbon dioxide and can better accelerate the conversion. Image courtesy Larry Curtiss, Argonne National Laboratory
One possible end product is methanol, a liquid fuel and the focus of a recent study conducted at the U.S. Department of Energy's (DOE) Argonne National Laboratory. The chemical reactions that make methanol from carbon dioxide rely on a catalyst to speed up the conversion, and Argonne scientists identified a new material that could fill this role. With its unique structure, this catalyst can capture and convert carbon dioxide in a way that ultimately saves energy.
They call it a copper tetramer.
It consists of small clusters of four copper atoms each, supported on a thin film of aluminum oxide. These catalysts work by binding to carbon dioxide molecules, orienting them in a way that is ideal for chemical reactions. The structure of the copper tetramer is such that most of its binding sites are open, which means it can attach more strongly to carbon dioxide and can better accelerate the conversion.
The current industrial process to reduce carbon dioxide to methanol uses a catalyst of copper, zinc oxide and aluminum oxide. A number of its binding sites are occupied merely in holding the compound together, which limits how many atoms can catch and hold carbon dioxide.
“With our catalyst, there is no inside,” said Stefan Vajda, senior chemist at Argonne and the Institute for Molecular Engineering and co-author on the paper. “All four copper atoms are participating because with only a few of them in the cluster, they are all exposed and able to bind.”
To compensate for a catalyst with fewer binding sites, the current method of reduction creates high-pressure conditions to facilitate stronger bonds with carbon dioxide molecules. But compressing gas into a high-pressure mixture takes a lot of energy.
The benefit of enhanced binding is that the new catalyst requires lower pressure and less energy to produce the same amount of methanol.
Carbon dioxide emissions are an ongoing environmental problem, and according to the authors, it's important that research identifies optimal ways to deal with the waste.
“We're interested in finding new catalytic reactions that will be more efficient than the current catalysts, especially in terms of saving energy,” said Larry Curtiss, an Argonne Distinguished Fellow who co-authored this paper.
Copper tetramers could allow us to capture and convert carbon dioxide on a larger scale–reducing an environmental threat and creating a useful product like methanol that can be transported and burned for fuel.
Of course the catalyst still has a long journey ahead from the lab to industry.
Potential obstacles include instability and figuring out how to manufacture mass quantities. There's a chance that copper tetramers may decompose when put to use in an industrial setting, so ensuring long-term durability is a critical step for future research, Curtiss said. And while the scientists needed only nanograms of the material for this study, that number would have to be multiplied dramatically for industrial purposes.
Meanwhile, the researchers are interested in searching for other catalysts that might even outperform their copper tetramer.
These catalysts can be varied in size, composition and support material, which results in a list of more than 2,000 potential combinations, Vajda said.
But the scientists don't have to run thousands of different experiments, said Peter Zapol, an Argonne physicist and co-author of this paper. Instead, they will use advanced calculations to make predictions, and then test the catalysts that seem most promising.
“We haven't yet found a catalyst better than the copper tetramer, but we hope to,” Vajda said. “With global warming becoming a bigger burden, it's pressing that we keep trying to turn carbon dioxide emissions back into something useful.”
For this research, the team used the Center for Nanoscale Materials as well as beamline 12-ID-C of the Advanced Photon Source, both DOE Office of Science User Facilities.
Curtiss said the Advanced Photon Source allowed the scientists to observe ultralow loadings of their small clusters, down to a few nanograms, which was a critical piece of this investigation.
###
The study, “Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures,” was published in the Journal of the American Chemical Society and was funded by the DOE's Office of Basic Energy Sciences. Co-authors also included researchers from the University of Freiburg and Yale University.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science. For more, visit http://www.
The Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…