An abundant and inexpensive water-splitting photocatalyst with low toxicity

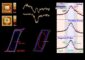
Figure 1 from the press release material. Electron microscope imagery of Sn3O4 catalyst. The synthesized material is a collection of microsized (one millionth of a meter) flaky crystals. Copyright : National Institute for Materials Science (NIMS)
Technology that allows the direct conversion of sunlight, an ultimate renewable energy, into chemical energies (i.e., fuels) that can be condensed and transported is not yet available. As such, solar energy is not ready at present to be utilized in place of conventional fossil and nuclear fuels.
Many water-splitting photocatalysts, such as titanium dioxide (TiO2), can decompose water and produce hydrogen fuel when absorbing ultraviolet light. However, due to their inability to absorb visible light, which accounts for more than half of solar energy, their practical use in the conversion of solar energy is limited.
While the development of new photocatalysts that can split water by absorbing visible light has been worked on globally, there are cost- and environment-related issues because many of the available photocatalysts contain expensive rare metals, such as tantalum, or high concentrations of lead, which is very toxic.
Led by Hideki Abe and Naoto Umezawa, researchers at Japan's National Institute for Materials Science (NIMS) recently discovered a novel photocatalyst by integrating both theoretical and experimental sciences.
The NIMS team searched for oxides containing divalent tin ions (Sn2+) based on the theoretical prediction that such substances may have an electronic structure conducive to water-splitting photocatalytic reactions under the presence of visible light.
As a result, they found a tin oxide, Sn3O4 (Sn2+2Sn4+O4), that is made up of divalent tin ions (Sn2+) and tetravalent tin ions (Sn4+). Their experiment revealed that this substance facilitates a water-splitting reaction leading to the generation of hydrogen when exposed to visible light which does not activate TiO2.
Since tin oxides are relatively non-toxic, inexpensive and abundant, they are widely used as transparent conductive materials. The discovery of the Sn3O4 catalyst is expected to greatly contribute to the reduction of environmental load and costs associated with hydrogen fuel production, and to the realization of a recycling-oriented society founded on the use of solar energy.
Results of this research will be published in the near future in the online version of Applied Materials & Interfaces, a journal issued by the American Chemical Society.
Associated links
Media Contact
More Information:
http://www.researchsea.comAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Peptides on Interstellar Ice
A research team led by Dr Serge Krasnokutski from the Astrophysics Laboratory at the Max Planck Institute for Astronomy at the University of Jena had already demonstrated that simple peptides…

A new look at the consequences of light pollution
GAME 2024 begins its experiments in eight countries. Can artificial light at night harm marine algae and impair their important functions for coastal ecosystems? This year’s project of the training…

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…