Molecules change shape when wet
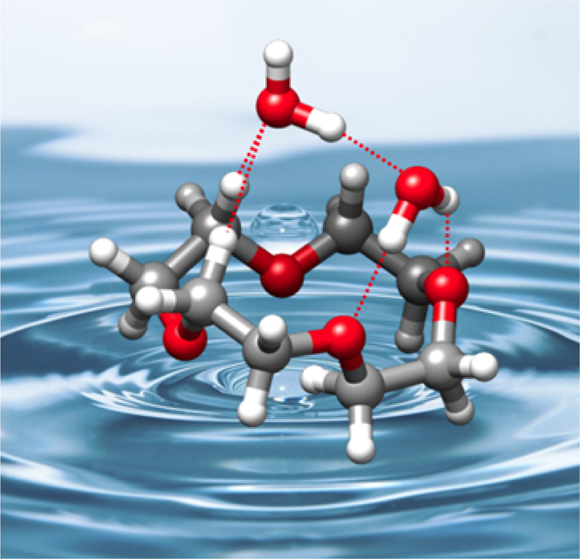
The preferred structure of a crown ether changes when water molecules bind to it (dashed lines). © C. Pérez et al.
In two recent publications in the Journal of Chemical Physics and in the Journal of Physical Chemistry Letters, researchers around Melanie Schnell from the Max Planck Institute for the Structure and Dynamics of Matter at CFEL and from the Hamburg Centre for Ultrafast Imaging (CUI) show that water promotes the reshaping of crown ethers and biphenyl molecules, two classes of chemically fascinating molecules.
Crown ethers are key systems in catalysis, separation and encapsulation processes, while biphenyl-based systems are employed in asymmetric synthesis and drug design.
Water has profound implications in our world. From its known – yet not fully understood – role in mediating protein folding dynamics and proton transport in membranes, water is a key player, influencing the mechanics of many biological and synthetic processes. In the present studies, the researchers use high-resolution rotational spectroscopy to investigate the structural effects that water induces in two types of molecular systems with different roles in the chemical realm.
Isolated micro-solvated molecules in the gas phase have become an appealing target to reveal the stepwise hydration of molecular systems. The Hamburg group of Melanie Schnell takes this route to reveal the effects that organic molecules undergo when the first water molecules bind around them, forming the so-called first solvation shell.
Crown ethers are cyclic molecules in a motif that resembles a crown. They have an extraordinary affinity for catching cations inside the crown. This function is either useful or harmful depending on the size of the crown and its consequent ability to bind smaller or larger cations such as potassium, sodium or lithium. Crown ethers are thus highly functional systems. In this work, the authors discovered that when binding to water, the crown ether’s preferred shape changes.
“The unexpected structural changes induced by the hydration of the crown ethers reveals new roads for host–guest interactions to take place,” says Cristóbal Pérez, postdoc at the MPSD and first author of this work. The expected efficiency for catching other species may be altered in the presence of water. Given the abundance of water at the molecular scale where many biological processes take place, this revelation is important for chemists working with catalysis where crown ethers are employed.
Biphenyl-based systems have a core consisting of two benzene (C6H6) rings connected via an axis. By overcoming a small energy barrier, the two rings are allowed to rotate with respect to each other. Clockwise and counterclockwise rotation generates mirror images of the same molecule that are not superimposed onto each other and can thus be termed chiral. The ability of identifying and assigning the mirror image forms of chiral molecules are key steps in drug design by the pharmaceutical industry. As an example, the biphenyl motif occurs in drug designs against tuberculosis.
In this study, it is revealed that when hydrated, the biphenyl system uses two water molecules to form what the authors call a “water-wire”. The water wire links the two rings of the biphenyl motif and consequently locks the position of the two rings with respect to each other. With this locking mechanism due to the presence of water, a measurable change in the angle between the rings is observed. “The observed phenomenon provides us with new clues to how water may mediate the interactions between a molecule and its potential receptor,” says Sérgio Domingos, postdoc at the MPSD and first author of this work. The observed structural changes induced by water are insightful on the role of hydration for the regulation of more complex biological processes taking place where water is the dominant medium.
Contact persons:
Dr. Cristóbal Pérez
Max Planck Institute for the Structure and Dynamics of Matter
Center for Free-Electron Laser Science
Luruper Chaussee 149
22761 Hamburg
Germany
+49 (0)40 8998-6233
cristobal.perez@mpsd.mpg.de
Dr. Sérgio Domingos
Max Planck Institute for the Structure and Dynamics of Matter
Center for Free-Electron Laser Science
Luruper Chaussee 149
22761 Hamburg
Germany
+49 (0)40 8998-6233
sergio.domingos@mpsd.mpg.de
PD Dr. Melanie Schnell
Max Planck Institute for the Structure and Dynamics of Matter
Center for Free-Electron Laser Science
Luruper Chaussee 149
22761 Hamburg
Germany
+49 (0)40 8998-6240
melanie.schnell@mpsd.mpg.de
Original publications:
C. Pérez, J. C. López, S. Blanco, and M. Schnell, “Water-Induced Structural Changes in Crown Ethers from Broadband Rotational Spectroscopy,” The Journal of Physical Chemistry Letters 7 (20), 4053-4058 (2016); DOI: 10.1021/acs.jpclett.6b01939
S. R. Domingos, C. Pérez, and M. Schnell, “Communication: Structural locking mediated by a water wire: A high-resolution rotational spectroscopy study on hydrated forms of a chiral biphenyl derivative,” The Journal of Chemical Physics 145 (16), 161103 (2016); DOI: 10.1063/1.4966584
https://dx.doi.org/10.1021/acs.jpclett.6b01939 Original publication
https://dx.doi.org/10.1063/1.4966584 Original publication
http://www.mpsd.mpg.de/en/research/irg/ccm Research group of PD Dr. Melanie Schnell
http://www.mpsd.mpg.de/en Max Planck Institute for the Structure and Dynamics of Matter
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















