Delicate balance regulates cell death in the intestinal epithelium
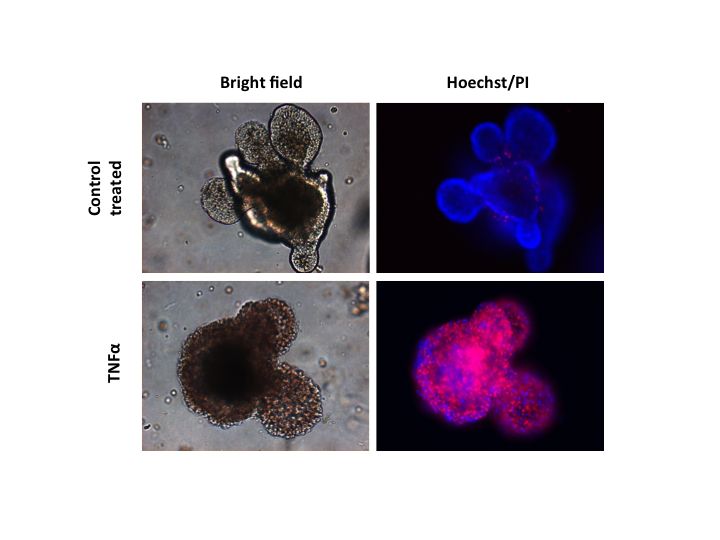
TNF-α-induced cell death in intestinal organoids grown in vitro in a cell culture dish. The accumulation of red cells confirms the cell death-promoting activity of TNF-α in intestinal epithelial cells.
The intestine is an important organ with a huge surface area about the size of up to one tennis court. Keeping the balance in the intestine between “inside” and “outside” – the microflora of the bowel and the body – is an extremely important, but also complicated task.
To efficiently absorb amino acids, fats and sugar, the surface is considerably enlarged by folds, villi and microvilli on the individual epithelial cells. In addition to this, the intestinal epithelium is only one cell layer thick, and therefore prone to damage. Generally, the body can deal with damage well, as the proliferation rate of the intestinal epithelium – the rate of regeneration through stem cells – is high. On the other hand this leads to an increased cancer risk as these stem cells are especially vulnerable to mutations.
Inflammatory bowel disease, such as Morbus Crohn or ulcerative colitis, acutely or chronically damages the intestinal epithelium. In these cases, the balance is so severely disturbed that the epithelium becomes massively damaged and eroded. This sets off a vicious cycle in which the intestinal flora has increased contact with the immune system, which in turn fuels the inflammatory processes even further.
Why the intestinal epithelium reacts so sensitively to damage caused by immune cells and why this delicate balance is so easily upset, was now investigated by the research team around Thomas Brunner, Professor of Biochemical Pharmacology at the University of Konstanz. The team could successfully demonstrate that particularly the intestinal epithelium, in contrast to other cells, directly reacts to a proinflammatory cytokine, the tumor necrosis factor-α (TNF-α), with excessive cell death. TNF-α promotes inflammation.
It is produced by activated macrophages (phagocytic cells), which play a central role in the immune defense. In most cells and tissues, TNF-α does not directly trigger the mechanism of programmed cell death. In the intestinal epithelium, however, this is much more likely. The reason for this are the molecules that can be recruited to the TNF-α receptor and then operate like a molecular switch, shifting the signal in one or the other direction.
These molecules, which Thomas Brunner and his team focus on, counteract cell death and are called IAPs (inhibitor of apoptosis proteins). They are so-called ubiquitin-ligases, enzymes, which change the function of other proteins via conjugation of ubiquitin chains. Three special molecules were investigated: cIAP1 and 2 (cellular inhibitor of apoptosis protein 1 and 2) as well as XIAP (X-chromosome-linked inhibitor of apoptosis protein). As the researchers had assumed, all three show only low expression levels in the intestinal epithelium – resulting in a lower protective function.
cIAP1 seems to play a crucial role in the regulation process. With the help of in vitro models of the gut – organoids derived from human stem cells or mouse intestinal crypts – the researchers could demonstrate that cells of the intestinal epithelium react extremely sensitively to TNF-α, while liver cells, for example, did not react at all or only to minor degree. This effect was much more pronounced if, in mice, the gene for the production of cIAP1 was deactivated. The same effect was observed if drugs, so-called IAP inhibitors, were added: the already sensitive epithelial cells become even more sensitive to TNF-α induced cell death.
To understand whether these cell death inhibitors are lowered in case of inflammatory diseases, or are not available at all, the researchers investigated TWEAK (TNF-related weak inducer of apoptosis). TWEAK is a molecule that is related to TNF-α, but is only a weak inducer of cell-death. However, it is known that it is involved in the development of inflammatory bowel disease. The researchers studied whether it makes the intestinal epithelium more sensitive to TNF-α. TWEAK seams to withdraw the cell death preventing IAP molecules from the receptor TNF-α with the result that the cells of the intestinal epithelium react highly sensitively to TNF- and die.
“We were able to demonstrate this not only in vitro or in intestinal organoids, but also in vivo. Cell death induction through TNF-α is amplified. Other studies have shown that blocking TWEAK has a positive therapeutic effect in models of inflammatory bowel disease,” Thomas Brunner explains. “Our study contributes on a molecular level to understand why the intestinal epithelium is so sensitive in the first place. Understanding these processes is the basis for a more targeted approach in which TNF-α is not simply blocked, but this sensitivity is modelled in a way that cell death induction becomes less pronounced in patients with inflammatory bowel disease, or can be redirected to support survival.”
Original publication:
Grabinger T, Bode KJ, Demgenski J, Seitz C, Delgado ME, Kostadinova F, Reinhold C, Etemadi N, Wilhelm S, Schweinlin M, Hänggi K, Knop J, Hauck C, Walles H, Silke J, Wajant H, Nachbur U, Wong WW, Brunner T.: „Inhibitor of Apoptosis Protein-1 Regulates Tumor Necrosis Factor-mediated Destruction of Intestinal Epithelial Cells”. Gastroenterology (23 November 2016)
https://www.ncbi.nlm.nih.gov/pubmed/27889570
Facts:
The research team around Professor Thomas Brunner investigated why particularly the intestinal epithelium reacts so sensitively to damage caused by immune cells.
The researchers could now demonstrate that the intestinal epithelium directly reacts to the proinflammatory tumor necrosis factor-α, as the protective function of “inhibitor of apoptosis proteins“ is lower than in other tissue.
Thomas Grabinger, Konstantin Bode and Carina Seitz were supported through doctoral scholarships of the DFG-funded research training group RTG 1331 as well as the cooperative graduate school “Promotionskolleg InViTe” (University of Konstanz, Albstadt-Sigmaringen University.
Contact
University of Konstanz
Communications and Marketing
Phone: + 49 7531 88-3603
E-Mail: kum@uni-konstanz.de
Media Contact
More Information:
http://www.uni-konstanz.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















