Research Shows How Water May Enhance Catalysis
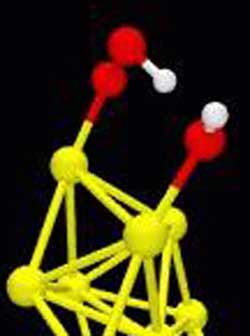
A snapshot of the reaction in which a water molecule enhances the transformation of carbon monoxide to carbon dioxide. Illustration of reaction turning carbon monoxide (CO) into carbon dioxide (CO2) using a water molecule (H20) to enhance the catalytic activity of an eight-atom nanocluster of gold. Color key: oxygen atoms = red; hydrogen atoms = white; carbon atoms = aquamarine; gold atoms = gold; and magnesium atoms = green.
Researchers at the Georgia Institute of Technology have uncovered important evidence that explains how water, usually an inhibitor of catalytic reactions, can sometimes promote them. The findings could lead to fewer constraints on reaction conditions potentially leading to the development of lower cost techniques for certain industrially important catalytic reactions. The results appear in the September 6, 2005 issue of Physical Review Letters.
“Normally, in most catalytic reactions, water can stop the reaction. It kills the catalyst,” said Uzi Landman, director of the Center for Computational Materials Science, Regents’ and Institute professor and Callaway chair of physics at Georgia Tech.
And that’s a big problem because ensuring that a reaction is water-free can add to production costs. Many catalytic reactions occur at high temperatures, which evaporates the water, said Landman. “However, any time that the reaction temperature is lowered and there’s humidity unfavorable effects may occur. You hope that when you heat the reaction up that the adsorbed water will come off, but sometimes it doesn’t. Sometimes the adsorption of water leads to an irreversible modification, such as oxidation, and deactivation of the catalyst. It’s poison; it poisons the catalyst,” he said.
In the late 1980’s, Japanese scientist Masatake Haruta discovered that small particles of gold (which is chemically inert in bulk form and normally not a catalyst) are chemically very reactive. He also found that water can promote this catalytic activity.
Since the late 1990’s, Landman’s group has been using advanced quantum mechanical computational methods to investigate how and why nanoclusters of gold act as chemical catalysts under dry conditions. This led to certain predictions that were verified experimentally by Ulrich Heiz’s group, who is now at the Technical University of Munich.
Earlier this year, the two groups co-authored a paper in the journal Science. It showed theoretical and experimental evidence of the role of charging on the catalytic activity of gold nanoclusters made of eight atoms when they are bonded to naturally occurring oxygen vacancy defects on a magnesia surface that supports the gold. In the recent Physical Review Letters paper, the Georgia Tech group has made theoretical predictions on how a single water molecule can catalytically enhance a low-temperature reaction that turns carbon monoxide into carbon dioxide.
Using computer simulations, Landman and post doctoral fellow Angelo Bongiorno, found that the water molecule enhances the binding of an oxygen molecule to an eight atom gold nanocluster, either free or supported on an undefective magnesia substrate. The water molecule catalytically activates the aforementioned oxidation reaction of carbon monoxide. In the earlier studies on gold nanoclusters, defects in the support surface were required to give the gold a slight negative charge. In this latest study, the presence of a water molecule makes that requirement unnecessary.
Here’s how it works: the structure of the water molecule, H-O-H, is such that the end with the oxygen atom has a slight negative charge, while the two hydrogen atoms are positively charged. In the quantum molecular dynamics simulation, the negatively charged oxygen side of the water molecule bonds to one of the gold atoms, leaving the positively charged hydrogens of the water molecule dangling. Subsequently, an oxygen molecule (made of two oxygen atoms) binds favorably to a neighboring gold atom of the cluster and gets a slight negative charge in the process.
This results in an adsorbed slightly negatively charged oxygen molecule near one of the positively charged hydrogen atoms of the adsorbed water molecule. Since, in chemistry, (as in love) opposites attract, the two get together. So the oxygen pulls a proton (a positively charged hydrogen) from the water molecule resulting in formation of a hydroperoxyl (OOH) group and a hydroxyl (OH).
Now, this relationship can’t last because the addition of the hydrogen to the oxygen molecule to form OOH weakens the bond between the two oxygen atoms. All it takes to break that bond is a carbon monoxide molecule approaching from the gas phase, which bonds to one of the oxygens of the OOH to form carbon dioxide. This leaves the proton to return to the hydroxyl to reform the water molecule. The product carbon dioxide desorbes readily from the surface, and the left over oxygen atom stays bonded to the gold. But this single oxygen atom is very active (as singles often are) and is easily led away when another carbon monoxide comes along to bond with it to make a second carbon dioxide molecule.
“This reaction opens the door to a completely new idea; that polar molecules, like water, or molecules that are good proton donors may show us new channels of reactivity,” said Landman. “We may be able to take other catalytic reactions and use water as a promoter under some selective conditions,” added Bongiorno.
“In the future, we want to test the effect of multiple water molecules to see if there is a limit to how many water molecules can enhance reactions. In this case, we used magnesium oxide as a substrate. We’d like to know if the effect limited to that substrate or will it work with others?,” the two researchers said.
(a) H20 (in red and white) approaches a nanocluster of eight gold atoms supported on a defect-free magnesium oxide surface.
(b) The oxygen atom of the H20 binds to the gold, leaving its positively charged hydrogen atoms dangling. Meanwhile an oxygen molecule (O2) bonds to the gold at another location with one of the oxygen attached directly to the gold cluster. The adsorbed oxygen molecule acquires a slight negative charge. An approaching CO molecule (red and green) is shown at the top.
(c) The dangling end of the oxygen molecule attracts a positively charged hydrogen atom off the H20 resulting in a hydroperoxyl group (OOH) and leaving a hydroxyl (OH) where H20 was.
(d) The carbon atom of the CO binds to the OOH, which causes the hydrogen atom to head on its way to join back with the OH to reform H20.
Media Contact
More Information:
http://www.icpa.gatech.eduAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Wildfire danger to increase due to climate change
WSL Institute for Snow and Avalanche Research (SLF) researchers expect an elevated wildfire danger in the Alpine Foreland from 2040 onwards due to changing meteorological conditions. The danger currently remains…
Advanced Brain Science Without Coding Expertise
Researchers at Helmholtz Munich and the LMU University Hospital Munich introduce DELiVR, offering a new AI-based approach to the complex task of brain cell mapping. The deep learning tool democratizes…
Gentle defibrillation for the heart
Using light pulses as a model for electrical defibrillation, Göttingen scientists developed a method to assess and modulate the heart function. The research team from the Max Planck Institute for…





















