Oxygen molecule survives to enormously high pressures: RUB publication in Physical Review Letters
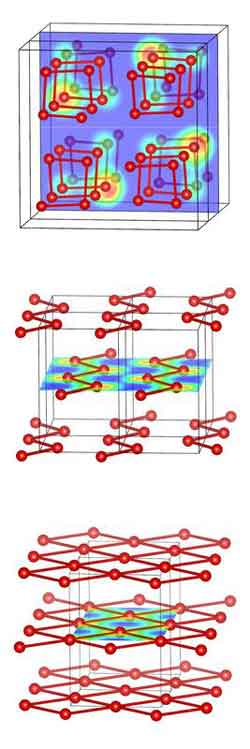
Structures of solid oxygen under high pressure: At 1.9 TPa, oxygen polymerizes and assumes a square spiral-like structure, which is semi-conducting (top). With increasing pressure, the polymer exhibits metallic properties (zig-zag chain-like phase, mid). Then, the structure changes into a metallic layer phase (bottom). The coloured areas represent the charge density in one layer of the structure. Figure: Jian Sun<br>
Using computer simulations, a RUB researcher has shown that the oxygen molecule (O2) is stable up to pressures of 1.9 terapascal, which is about nineteen million times higher than atmosphere pressure. Above that, it polymerizes, i.e. builds larger molecules or structures.
“This is very surprising” says Dr. Jian Sun from the Department of Theoretical Chemistry. “Other simple molecules like nitrogen or hydrogen do not survive such high pressures.” In cooperation with colleagues from University College London, the University of Cambridge, and the National Research Council of Canada, the researcher also reports that the behaviour of oxygen with increasing pressure is very complicated. It's electrical conductivity first increases, then decreases, and finally increases again. The results are published in Physical Review Letters.
Weaker bonds, greater stability
The oxygen atoms in the O2 molecule are held together by a double covalent bond. Nitrogen (N2), on the other hand, possesses a triple bond. “You would think that the weaker double bond is easier to break than the triple bond and that oxygen would therefore polymerize at lower pressures than nitrogen” says Sun. “We found the opposite, which is astonishing at first sight.”
Coming together when pressure increases
However, in the condensed phase when pressure increases, the molecules become closer to each other. The research team suggests that, under these conditions, the electron lone pairs on different molecules repel one another strongly, thus hindering the molecules from approaching each other. Since oxygen has more lone pairs than nitrogen, the repulsive force between these molecules is stronger, which makes polymerization more difficult. However, the number of lone pairs cannot be the only determinant of the polymerization pressure. “We believe that it is a combination of the number of lone pairs and the strength of the bonds between the atoms”, says Sun.
The many structures of oxygen
At high pressures, gaseous molecules such as hydrogen, carbon monoxide, or nitrogen polymerize into chains, layers, or framework structures. At the same time they usually change from insulators to metals, i.e. they become more conductive with increasing pressure. The research team, however, showed that things are more complicated with oxygen. Under standard conditions, the molecule has insulating properties. If the pressure increases, oxygen metallises and becomes a superconductor. With further pressure increase, its structure changes into a polymer and it becomes semi-conducting. If the pressure rises even more, oxygen once more assumes metallic properties, meaning that the conductivity goes up again. The metallic polymer structure finally changes into a metallic layered structure.
Inside planets
“The polymerization of small molecules under high pressure has attracted much attention because it helps to understand the fundamental physics and chemistry of geological and planetary processes” explains Sun. “For instance, the pressure at the centre of Jupiter is estimated to be about seven terapascal. It was also found that polymerized molecules, like N2 and CO2, have intriguing properties, such as high energy densities and super-hardness.” Dr. Jian Sun joined the RUB-research group of Prof. Dr. Dominik Marx as a Humboldt Research Fellow in 2008 to work on vibrational spectroscopy of aqueous solutions. In parallel to this joint work in “Solvation Science” he developed independent research interests into high pressure chemical physics as an Early Career Researcher.
Bibliographic record
J. Sun, M. Martinez-Canales, D.D. Klug, C.J. Pickard, R.J. Needs (2012): Persistence and eventual demise of oxygen molecules at terapascal pressures, Physical Review Letters, doi: 10.1103/PhysRevLett.108.045503
Further information
Dr. Jian Sun, Department of Theoretical Chemistry, Faculty of Chemistry and Biochemistry at the Ruhr-Universität, 44780 Bochum, Tel.: +49/234/32-22121
jian.sun@theochem.rub.de
Click for more
Department of Theoretical Chemistry
http://www.theochem.rub.de/home.en.html
Editorial journalist
Dr. Julia Weiler
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















