NASA Research Gives Guideline for Future Alien Life Search
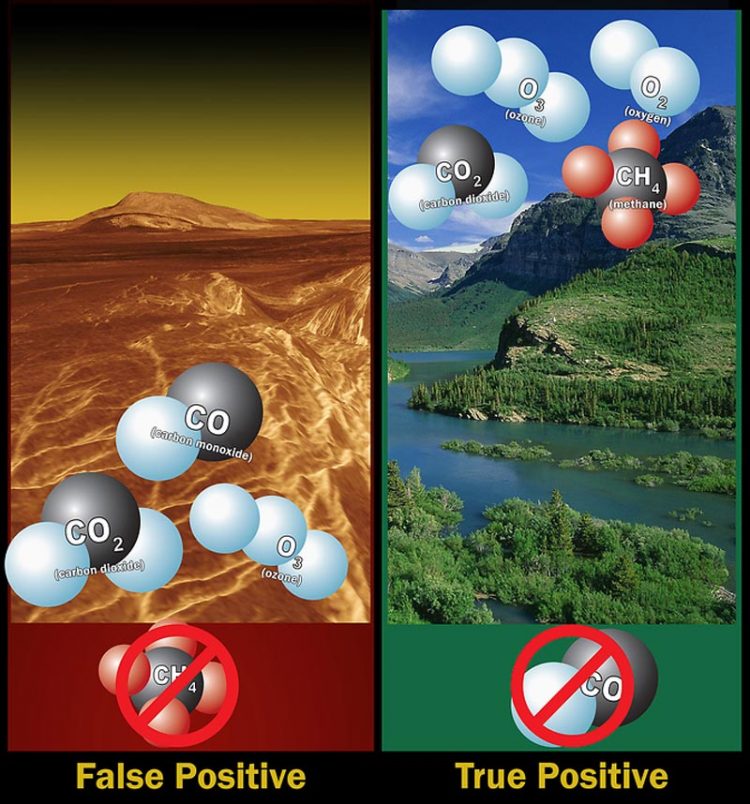
Left: Ozone molecules in a planet's atmosphere could indicate biological activity, but ozone, carbon dioxide and carbon monoxide -- without methane, is likely a false positive. Right: Ozone, oxygen, carbon dioxide and methane -- without carbon monoxide, indicate a possible true positive. Image Credit: NASA
The researchers carefully simulated the atmospheric chemistry of alien worlds devoid of life thousands of times over a period of more than four years, varying the atmospheric compositions and star types. “When we ran these calculations, we found that in some cases, there was a significant amount of ozone that built up in the atmosphere, despite there not being any oxygen flowing into the atmosphere,” said Shawn Domagal-Goldman of NASA's Goddard Space Flight Center in Greenbelt, Maryland. “This has important implications for our future plans to look for life beyond Earth.”
Methane is a carbon atom bound to four hydrogen atoms. On Earth, much of it is produced biologically (flatulent cows are a classic example), but it can also be made inorganically; for example, volcanoes at the bottom of the ocean can release the gas after it is produced by reactions of rocks with seawater.
Ozone and oxygen were previously thought to be stronger biosignatures on their own. Ozone is three atoms of oxygen bound together. On Earth, it is produced when molecular oxygen (two oxygen atoms) and atomic oxygen (a single oxygen atom) combine, after the atomic oxygen is created by other reactions powered by sunlight or lightning. Life is the dominant source of the molecular oxygen on our planet, as the gas is produced by photosynthesis in plants and microscopic, single-cell organisms. Because life dominates the production of oxygen, and oxygen is needed for ozone, both gases were thought to be relatively strong biosignatures. But this study demonstrated that both molecular oxygen and ozone can be made without life when ultraviolet light breaks apart carbon dioxide (a carbon atom bound to two oxygen atoms). Their research suggests this non-biological process could create enough ozone for it to be detectable across space, so the detection of ozone by itself would not be a definitive sign of life.
“However, our research strengthens the argument that methane and oxygen together, or methane and ozone together, are still strong signatures of life,” said Domagal-Goldman. “We tried really, really hard to make false-positive signals for life, and we did find some, but only for oxygen, ozone, or methane by themselves.” Domagal-Goldman and Antígona Segura from the Universidad Nacional Autónoma de México in Mexico City are lead authors of a paper about this research, along with astronomer Victoria Meadows, geologist Mark Claire, and Tyler Robison, an expert on what Earth would look like as an extrasolar planet. The paper appeared in the Astrophysical Journal Sept. 10, and is available online.
Methane and oxygen molecules together are a reliable sign of biological activity because methane doesn't last long in an atmosphere containing oxygen-bearing molecules. “It's like college students and pizza,” says Domagal-Goldman. “If you see pizza in a room, and there are also college students in that room, chances are the pizza was freshly delivered, because the students will quickly eat the pizza. The same goes for methane and oxygen. If both are seen together in an atmosphere, the methane was freshly delivered because the oxygen will be part of a network of reactions that will consume the methane. You know the methane is being replenished. The best way to replenish methane in the presence of oxygen is with life. The opposite is true, as well. In order to keep the oxygen around in an atmosphere that has a lot of methane, you have to replenish the oxygen, and the best way to do that is with life.”
Scientists have used computer models to simulate the atmospheric chemistry on planets beyond our solar system (exoplanets) before, and the team used a similar model in its research. However, the researchers also developed a program to automatically compute the calculations thousands of times, so they could see the results with a wider range of atmospheric compositions and star types.
In doing these simulations, the team made sure they balanced the reactions that could put oxygen molecules in the atmosphere with the reactions that might remove them from the atmosphere. For example, oxygen can react with iron on the surface of a planet to make iron oxides; this is what gives most red rocks their color. A similar process has colored the dust on Mars, giving the Red Planet its distinctive hue. Calculating the appearance of a balanced atmosphere is important because this balance would allow the atmosphere to persist for geological time scales. Given that planetary lifetimes are measured in billions of years, it's unlikely astronomers will happen by chance to be observing a planet during a temporary surge of oxygen or methane lasting just thousands or even millions of years.
It was important to make the calculations for a wide variety of cases, because the non-biological production of oxygen is subject to both the atmospheric and stellar environment of the planet. If there are a lot of gases that consume oxygen, such as methane or hydrogen, then any oxygen or ozone produced will be destroyed in the atmosphere. However, if the amount of oxygen-consuming gases is vanishingly small, the oxygen and the ozone might stick around for a while. Likewise, the production and destruction of oxygen, ozone, and methane is driven by chemical reactions powered by light, making the type of star important to consider as well. Different types of stars produce the majority of their light at specific colors. For example, massive, hot stars or stars with frequent explosive activity produce more ultraviolet light. “If there is more ultraviolet light hitting the atmosphere, it will drive these photochemical reactions more efficiently,” said Domagal-Goldman. “More specifically, different colors (or wavelengths) of ultraviolet light can affect oxygen and ozone production and destruction in different ways.”
Astronomers detect molecules in exoplanet atmospheres by measuring the colors of light from the star the exoplanet is orbiting. As this light passes through the exoplanet's atmosphere, some of it is absorbed by atmospheric molecules. Different molecules absorb different colors of light, so astronomers use these absorption features as unique “signatures” of the type and quantity of molecules present.
“One of the main challenges in identifying life signatures is to distinguish between the products of life and those compounds generated by geological processes or chemical reactions in the atmosphere. For that we need to understand not only how life may change a planet but how planets work and the characteristics of the stars that host such worlds”, said Segura.
The team plans to use this research to make recommendations about the requirements for future space telescopes designed to search exoplanet atmospheres for signs of alien life. “Context is key – we can't just look for oxygen, ozone, or methane alone,” says Domagal-Goldman. “To confirm life is making oxygen or ozone, you need to expand your wavelength range to include methane absorption features. Ideally, you’d also measure other gases like carbon dioxide and carbon monoxide [a molecule with one carbon atom and one oxygen atom]. So we're thinking very carefully about the issues that could trip us up and give a false-positive signal, and the good news is by identifying them, we can create a good path to avoid the issues false positives could cause. We now know which measurements we need to make. The next step is figuring out what we need to build and how to build it.”
The research was funded in part by the NASA Astrobiology Institute's (NAI) Virtual Planetary Laboratory (VPL). The NAI is administered by NASA's Ames Research Center in Mountain View, California, and funded as part of the NASA Astrobiology Program at NASA Headquarters, Washington. The VPL is based at the University of Washington, and comprises researchers at 20 institutions working to understand how telescopic observations and modeling studies can determine if exoplanets are able to support life, or had life in the past. Additional support for the research was provided by the NASA Postdoctoral Program, managed by Oak Ridge Associated Universities.
The team represented an international collaboration that included researchers from NASA Goddard, NASA Ames, the NAI/VPL, the Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México, Mexico; the University of St. Andrews, St. Andrews, Scotland; and the University of Washington, Seattle.
For more information about the NASA Astrobiology Institute, visit:
The research paper is available online at:
http://stacks.iop.org/0004-637X/792/90
William Steigerwald
NASA's Goddard Space Flight Center, Greenbelt, Maryland
Gabriela Frias
Universidad Nacional Autonoma de Mexico, Mexico City
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















