An Incredible Shrinking Material
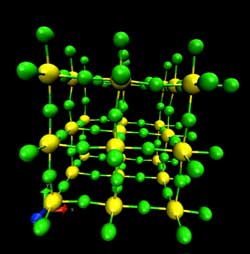
Heat causes the atoms in ScF3 to vibrate, as captured in this snapshot from a simulation. Fluorine atoms are in green while scandium atoms are in yellow. <br>[Credit: Caltech/C. Li et al.]<br>
They shrink when you heat 'em. Most materials expand when heated, but a few contract. Now engineers at the California Institute of Technology (Caltech) have figured out how one of these curious materials, scandium trifluoride (ScF3), does the trick—a finding, they say, that will lead to a deeper understanding of all kinds of materials.
The researchers, led by graduate student Chen Li, published their results in the November 4 issue of Physical Review Letters (PRL).
Materials that don't expand under heat aren't just an oddity. They're useful in a variety of applications—in mechanical machines such as clocks, for example, that have to be extremely precise. Materials that contract could counteract the expansion of more conventional ones, helping devices remain stable even when the heat is on.
“When you heat a solid, most of the heat goes into the vibrations of the atoms,” explains Brent Fultz, professor of materials science and applied physics and a coauthor of the paper. In normal materials, this vibration causes atoms to move apart and the material to expand. A few of the known shrinking materials, however, have unique crystal structures that cause them to contract when heated, a property called negative thermal expansion. But because these crystal structures are complicated, scientists have not been able to clearly see how heat—in the form of atomic vibrations—could lead to contraction.
But in 2010 researchers discovered negative thermal expansion in ScF3, a powdery substance with a relatively simple crystal structure. To figure out how its atoms vibrated under heat, Li, Fultz, and their colleagues used a computer to simulate each atom's quantum behavior. The team also probed the material's properties by blasting it with neutrons at the Spallation Neutron Source at Oak Ridge National Laboratory (ORNL) in Tennessee; by measuring the angles and speeds with which the neutrons scattered off the atoms in the crystal lattice, the team could study the atoms' vibrations. The more the material is heated the more it contracts, so by doing this scattering experiment at increasing temperatures, the team learned how the vibrations changed as the material shrank.
The results paint a clear picture of how the material shrinks, the researchers say. You can imagine the bound scandium and fluorine atoms as balls attached to one another with springs. The lighter fluorine atom is linked to two heavier scandium atoms on opposite sides. As the temperature is cranked up, all the atoms jiggle in many directions. But because of the linear arrangement of the fluorine and two scandiums, the fluorine vibrates more in directions perpendicular to the springs. With every shake, the fluorine pulls the scandium atoms toward each other. Since this happens throughout the material, the entire structure shrinks.
The surprise, the researchers say, was that in the large fluorine vibrations, the energy in the springs is proportional to the atom's displacement—how far the atom moves while shaking—raised to the fourth power, a behavior known as a quartic oscillation. Most materials are dominated by quadratic (or harmonic) oscillations—characteristic of the typical back-and-forth motion of springs and pendulums—in which the stored energy is proportional to the square of the displacement.
“A nearly pure quantum quartic oscillator has never been seen in atom vibrations in crystals,” Fultz says. Many materials have a little bit of quartic behavior, he explains, but their quartic tendencies are pretty small. In the case of ScF3, however, the team observed the quartic behavior very clearly. “A pure quartic oscillator is a lot of fun,” he says. “Now that we've found a case that's very pure, I think we know where to look for it in many other materials.” Understanding quartic oscillator behavior will help engineers design materials with unusual thermal properties. “In my opinion,” Fultz says, “that will be the biggest long-term impact of this work.”
The other authors of the PRL paper, “The structural relationship between negative thermal expansion and quartic anharmonicity of cubic ScF3,” are former Caltech postdoctoral scholars Xiaoli Tang and J. Brandon Keith; Caltech graduate students Jorge Muñoz and Sally Tracy; and Doug Abernathy of ORNL. The research was supported by the Department of Energy.
Click here: http://www.youtube.com/user/caltech#p/u/0/l46Kgm5u3Nw
for the video of the simulation.
Written by Marcus Woo
Deborah Williams-Hedges
626-395-3227
debwms@caltech.edu
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















