Highly charged ions
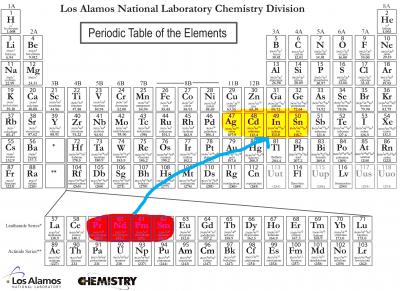
The best highly charged ions seem to be atoms such as Nd, Pr, and Sm that can be ionized to have the same number of electrons as four successive elements -- Ag, Cd, In, and Sn -- in another part of the periodic table. Credit: Adapted from Los Alamos figure
The world is mostly neutral. That is, most of the atoms in our environment are electrically neutral. The number of electrons in the outer parts of atoms equals the number of protons at the centers of atoms. As one or more electrons are plucked away from the atoms, the remaining electrons feel a much stronger positive pull from the nucleus.
This enhanced pull, causing the atoms to shrink in size, ensures that those electrons are less vulnerable to the distractions of their environment, making them potentially valuable for next-generation atomic clocks, for quantum information schemes (where the loss of quantum coherence in qubits is a paramount danger), and for experiments trying to detect slight variations in the fine structure constant, the parameter that sets the overall strength of the electromagnetic force.
A new theoretical study conducted by JQI adjunct fellow Marianna Safronova and her colleagues from groups around the world (1) provides the best yet study of how highly charged ions could be used for atomic timekeeping and for processing quantum information. They identify 10 such ions—for instance, samarium-14+ and neodymium-10+—along with estimates of ion properties experimenters need to know before beginning their work, things such as the expected lifetimes and internal energy levels for the excited states of the ions.
WORKING WITH HIGHLY CHARGED IONS
Charged-up atoms are hard to produce and control. At one of the facilities dedicated to this purpose, the Electron Beam Ion Trap (EBIT) at the Lawrence Livermore National Lab, a beam of electrons intercepts a beam of atoms, ionizing the atoms at they go. In this way charge states all the way up to +92 (fully ionized uranium atoms) have been achieved. The trick is to store such charged ions and to cool them to low temperatures. In the kind of atomic environments typical of atomic clocks or quantum computers, low temperature means small ion motion, which makes for better spectroscopy.
Spectroscopy, the measurement of the energy of atomic transitions, is all important for the applications mentioned above—clocks, quantum computing, and testing the constants of nature. Currently the world's time standard is pegged to a particular microwave transition in cesium-133 atoms.. Even higher precision and better clocks will result from the use of transitions in the optical range.
One problem of working with highly charged ions is that the gaps in the energy levels are too great. This is because when you ionize an atom, its energy levels get further apart. The light emanating from transitions in these ions is at too great a frequency, often in the ultraviolet part of the electromagnetic spectrum. Precision control and manipulation of ions needed for clocks and quantum information is harder or presently impossible to do in that UV or even x-ray energy regime. So in choosing ion candidates, it is important to search for transitions in the optical or near-optical range.
Another criterion is that the ions should be able to attain semi-stable excited states. A third criterion is that the characteristic transition is not between the states from the same electronic configuration. Such transitions do not have enhanced sensitivity to the study whether the fine structure constant is changing over time.. A fourth criterion is that the final ionic state should not be a radioactive substance, thus reducing handling problems.
Safronova and her colleagues, using these criteria and a state-of-the-art methods that they developed to study atoms arrived at their list of ten worthy ion species. They publish their results in the 18 July 2014 issue of Physical Review Letters (2).
Not the least part of their achievement is the authors' specification of the frequencies to be expected from the critical transitions in the candidate ions. It's hard enough to calculate the transition energies of un-ionized atoms, but even harder with these particular highly charged ions. They calculated the frequencies for their select ions and then compared (where appropriate) with the values observed in the lab. The difference, generally less than 1%, should reassure experimenters that Safronova and her colleagues can accurately predict properties of ions where no experimental data are available. .
CLOCKWORK
The use of highly charged ions might result in more accurate clocks since the atoms will be more immune from interference from nearby electric or magnetic fields. But aren't atomic clocks already good? They are. In January 2014, physicists at NIST-Boulder announced the creation of an atomic clock that sets records for accuracy and stability, at the 6 parts-per-10^-18 level. This clock uses strontium atoms, and the observed transition is reported to an astounding degree of explicitness. The energy corresponds to a wave with a frequency of 429,228,004,229, 870.0(1.1) Hz.
If, however, we could go just a bit further, to the level of 10^-19, then important physics tests might be possible, including the chance to determine whether the fine structure constant (denoted by the Greek letter alpha), is changing in time or space. Astronomical data has been interpreted by some to suggest that alpha is changing at a small level. At the finer level of precision offered by highly charged ions, terrestrial tests of alpha's constancy could be carried out.
Other potential uses for atomic clocks emerge in areas where high precision is critical: geodesy, hydrology, navigation, and even the deep tracking of spacecraft. Safronova singles out the potential for quantum computing: “The highly charge ions we recommend,” she said, “present a completely unexplored resource for quantum information owing to their unique atomic properties and their potential for reducing the sensitivity to troubling decoherence effects.”
(1) Safronova is an adjunct fellow of the Joint Quantum Institute (NIST/University of Maryland) and is also a professor at the University of Delaware. Her colleagues hail from Delaware, the University of New South Wales, the University of Nevada, the University of Notre Dame, the Petersburg Nuclear Physics Institute, and the St. Petersburg Electrotechnical University.
(2) “Highly charged ions for atomic clocks, quantum information, and search for alpha variation,” M.S. Safronova, V.A. Dzuba, V.V. Flambaum, U.I. Safronova, S.G. Porsev, and M.G. Kozlov, Phys. Rev. Lett. 113, 030801 – Published 16 July 2014. DOI: http://dx.doi.org/10.1103/PhysRevLett.113.030801
Marianna Safronova, msafrono@udel.edu
Press contact at JQI: Phillip F. Schewe, pschewe@umd.edu, 301-405-0989. http://jqi.umd.edu/
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















