A Step Closer to a Malaria Vaccine
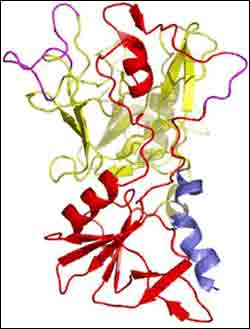
A "ribbon diagram" that illustrates the AMA1 segment’s molecular structure
An international team of scientists that includes a researcher from the U.S. Department of Energy’s Brookhaven National Laboratory has determined the three-dimensional molecular structure of a promising malaria-vaccine component. This research may help lead to a successful vaccine for the disease, which currently infects approximately 400 million people worldwide and kills about two million people each year — mostly children. The study is described in the August 29, 2005, online edition of the Proceedings of the National Academy of Sciences.
“The high number of deaths from malaria is partly due to the malaria parasite’s acquired resistance to traditional treatments,” said the study’s lead researcher, biologist Adrian Batchelor of the University of Maryland School of Pharmacy. “The parasite is a highly complex organism that develops through different life-cycle stages. This has allowed it to evade the immune system and makes creating a comprehensive vaccine a difficult task.”
Malaria vaccines to date have not been entirely effective, only able to temporarily suppress the disease. A complete, fully protective malaria vaccine will likely consist of several components, each only partially successful at fighting malaria on its own. The potential “part” studied here is a protein known as “Apical Membrane Antigen 1” (AMA1), a protein found on the cell membrane of Plasmodium falciparum, the parasite that causes the most deadly form of malaria.
A vaccine based on AMA1 has a good chance for success because AMA1 is produced, or “expressed,” in two critical parasite life-cycle stages. However, across different malaria strains, AMA1 can have many slight structure variations, called “polymorphisms.” These variations are problematic for vaccine development. Locating the polymorphic sites on AMA1 by determining its structure is essential to understanding how those sites might impact the development of a vaccine.
The research team focused on a particular segment of AMA1. They studied it using x-rays at Brookhaven’s National Synchrotron Light Source (NSLS), a facility that produces x-ray, ultraviolet, and infrared light for research. The x-ray analysis showed that the segment consists of two distinct regions, called domains, and further revealed unusual features: long molecular loops extending outward from the center of one domain. These loops form a “scaffold” for attached amino acids, which can mutate without affecting the function of AMA1. These mutations produce the different AMA1 polymorphisms.
“We think that these polymorphism-bearing loops serve a purpose, such as ‘protecting’ a critical portion of AMA1 from attack by human antibodies,” said Batchelor. “In fact, the AMA1 loops surround a molecular ‘trough’ that we suspect may be responsible for tethering malaria parasites to human red blood cells.”
Biophysicist Michael Becker, the Brookhaven scientist involved, said, “It feels good to contribute to efforts in the fight against malaria, as it’s a critically important disease to eradicate, especially for underprivileged regions of the world, and it is scientifically fascinating. Regarding Brookhaven’s role, it’s the indivisible wedding of science and technology at facilities such as the NSLS — and hopefully at the planned upgraded facility, NSLS-II — that provide us with the tools to pursue and create new science that can solve critical human problems in the real world.”
The researchers plan to build on this research by attempting to identify compounds that will fit into the trough and could prevent the malaria parasite from binding to red blood cells. They will also try to determine if there are non-polymorphic regions of AMA1 that could function as a vaccine.
This study also included scientists from the Commonwealth Scientific and Industrial Research Organization and La Trobe University, both located in Australia. It was supported by the Office of Basic Energy Sciences and the Office of Biological and Environmental Research, both within the U.S. Department of Energy’s Office of Science, as well as the National Center for Research Resources within the National Institutes of Health, and the University of Maryland School of Pharmacy.
Related Research
For another recent announcement about a protein structure that may be important in developing a malaria vaccine, see this release. This structure was also determined at the NSLS at Brookhaven Lab.
Media Contact
More Information:
http://www.bnl.govAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















