Injection prevents blinding blood vessel growth in mice

Researchers at Johns Hopkins’ Wilmer Eye Institute and Regeneron Pharmaceuticals have identified an experimental medicine that stops the blinding blood vessel growth associated with diabetic eye diseases and possibly macular degeneration in laboratory mice.
By injecting a fused protein called VEGF-TRAP (R1R2) into the eyes or bloodstreams of mice, scientists halted new blood vessel growth in the rodents’ eyes and stopped existing blood vessels from leaking. Study results were published recently in the Journal of Cellular Physiology.
VEGF-TRAP was designed to antagonize vascular endothelial growth factor (VEGF), a substance naturally produced in the body that promotes blood vessel formation. Released by the retina (light-sensitive tissue in back of the eye) when normal blood vessels are damaged by disease, VEGF turns on its receptor, igniting a chain reaction that culminates in new blood vessel growth. However, the backup blood vessels are faulty; they leak, bleed and encourage scar tissue that detaches the retina, resulting in severe loss of vision. Such growth is the hallmark of diabetic retinopathy, the leading cause of blindness among young people in developed countries. It’s also believed that VEGF contributes to abnormal blood vessel growth from the choroid layer of the eye into the retina, similar to what occurs during the wet or neovascular form of age-related macular degeneration.
VEGF-TRAP contains a portion of two receptors for VEGF, which, hooked together, form a sponge that soaks up VEGF before it causes additional damage. Scientists tested VEGF-TRAP in two groups of laboratory mice. In the first group, which had a rupture of Bruch’s membrane (the layer between the retina and the choroid), they injected VEGF-TRAP directly into the rodents’ eyes. In a second group of mice genetically engineered to express VEGF in the retina, they gave the mice injections of VEGF-TRAP under the skin.
Mice treated with either system-wide or local injections of VEGF-TRAP had significantly less new blood vessel growth. Also, VEGF-TRAP blocked leaking of blood vessels usually caused by VEGF, the major problem in patients with macular edema or swelling. This indicates that unlike some large molecules now being tested in patients, VEGF-TRAP does not have to be injected directly into the eye. Injections can be given under the skin, and VEGF-TRAP can, through circulation, exert a strong therapeutic effect in the eye against abnormal blood vessel formation. No side effects were identified.
Peter A. Campochiaro, M.D., senior study author and professor of ophthalmology and neuroscience, says if clinical studies demonstrate similar properties, he hopes that eventually patients could self-administer injections at home similar to insulin shots taken by diabetics. Unlike other treatments for macular degeneration and diabetic retinopathy, which require treatment of one eye at a time, the shot could take care of both eyes at once.
“Our data suggest that VEGF-TRAP deserves consideration as a potential treatment for two complications of diabetic retinopathy — new blood vessel growth and macular edema,” Campochiaro says. “It had long-lasting effects and did not cause complications.”
Circulating levels of VEGF-TRAP or other anti-VEGF medications could have many additional health benefits, Campochiaro says, as abnormal blood vessel growth has been implicated in tumor growth, hardening of the arteries and arthritis. A clinical trial sponsored by Regeneron Pharmaceuticals is being planned to assess the medication’s effects in people with diabetic retinopathy and macular edema, and in patients with wet age-related macular degeneration.
The study was supported by grants from the Public Health Service, the Foundation Fighting Blindness, Research to Prevent Blindness and Dr. and Mrs. William Lake. Coauthors were Yumiko Saishin, Kyoichi Takahashi and Raquel Lima E. Silva of Hopkins, and Donna Hylton, John S. Rudge and Stanley J. Wiegand of Regeneron, Tarrytown, N.Y.
###
Saishin, Yoshitsugu, et al, “VEGF-TRAP Suppresses Choroidal Neovascularization and VEGF-Induced Breakdown of the Blood-Retinal Barrier,” Journal of Cellular Physiology, May 2003, Vol. 195, pages 241-248.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Wildfire danger to increase due to climate change
WSL Institute for Snow and Avalanche Research (SLF) researchers expect an elevated wildfire danger in the Alpine Foreland from 2040 onwards due to changing meteorological conditions. The danger currently remains…
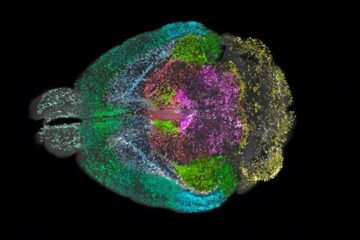
Advanced Brain Science Without Coding Expertise
Researchers at Helmholtz Munich and the LMU University Hospital Munich introduce DELiVR, offering a new AI-based approach to the complex task of brain cell mapping. The deep learning tool democratizes…
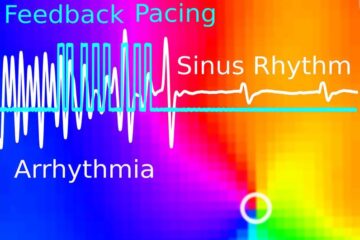
Gentle defibrillation for the heart
Using light pulses as a model for electrical defibrillation, Göttingen scientists developed a method to assess and modulate the heart function. The research team from the Max Planck Institute for…





















