New protease inhibitor held HIV at undetectable levels for four years

A study from The Feinberg School of Medicine has shown that the protease inhibitor lopinavir/ritonavir (Kaletra®) suppressed HIV to undetectable levels and was well tolerated through four years of treatment in patients who had not previously received antiretroviral therapy.
To date in the Kaletra® study, none of the patients has developed resistance to Kaletra® or other protease inhibitors. Kaletra® is thus far the only protease inhibitor for which resistance has not been observed in patients receiving it as an initial therapy.
Robert L. Murphy, M.D., professor of medicine at the Feinberg School and director of HIV/AIDS clinical research at Northwestern University, presented results of the study today at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy.
“Maintaining antiviral suppression to undetectable levels over the long term is crucial to patients’ success,” Murphy said.
“Drugs such as Kaletra®, which have demonstrated high rates of viral suppression over several years, remain important options for initial therapy. Viral suppression helps prolong the time to development of resistance, which is an important cause of treatment failure,” Murphy said.
In this ongoing phase II study, 100 patients who had not previously received antiretroviral therapy were given one of three doses of Kaletra® in addition to the nucleoside analogues stavudine and lamivudine.
After 48 weeks, therapy for all patients was converted to the same dose of Kaletra® with stavudine and lamivudine. Of the original group of patients, 72 remained in the study through four years; seven of the 28 patients who discontinued therapy did so because of adverse events attributed to Kaletra®. All 72 patients maintained an undetectable HIV viral load of less than 400 copies per milliliter, and their CD4 counts increased consistently from the beginning of the study over the four-year period (mean increase of 416 cells per cubic millimeter).
Kaletra® is receiving accelerated approval status in the United States and in several other countries and remains under review by the Food and Drug Administration for approval.
Media Contact
More Information:
http://www.nwu.edu/All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles
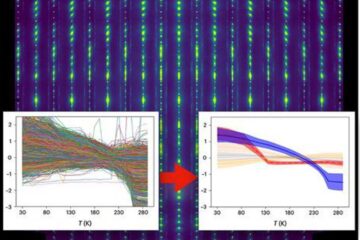
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
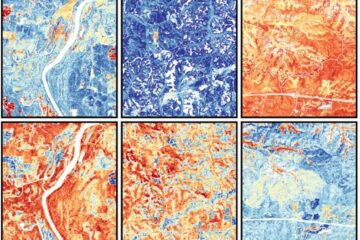
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















