Drug Shows Promise for the First Time Against Metastatic Melanoma of the Eye
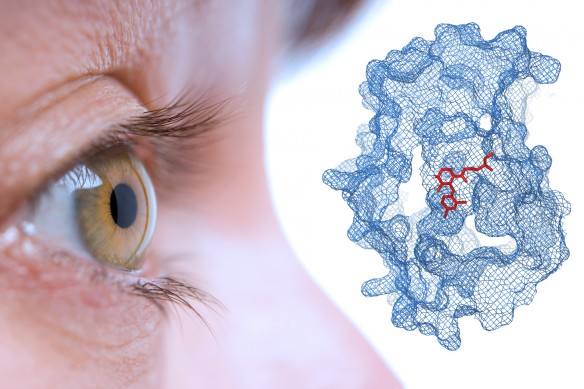
The melanoma-treating drug, selumetinib (red) bound to its protein target MEK (blue). (Image by Dr. Lucky Tran.)
Results from a multicenter clinical trial show that a new drug called selumetinib increases progression-free survival, the length of time during and after treatment that a patient with metastases lives with the disease without it progressing. The findings were published in the online edition of JAMA, the Journal of the American Medical Association.
“Although the effects of the drug were modest, we now know that we can influence the course of the disease, and we expect to build on this success with other drugs, including some already in development,” said senior author, Gary K. Schwartz, MD, professor of medicine and chief of hematology/oncology at NewYork-Presbyterian/Columbia University Medical Center and associate director of its Herbert Irving Comprehensive Cancer Center. (At the time of the trial, Dr. Schwartz was chief of the melanoma and sarcoma service at Memorial Sloan Kettering Cancer Center in New York.)
Uveal melanoma is a cancer of the iris, ciliary body, or choroid—structures in the eye collectively known as the uvea. Uveal melanoma (which is biologically distinct from skin melanoma) arises from the uvea’s melanocytes, the pigment cells that give the eye its color. Once the disease has spread—most metastases appear in the liver—existing treatments are largely ineffective. About 1,500 cases of uveal melanoma occur in the United States each year, usually among older adults. The median survival rate for patients with metastatic uveal melanoma is 12 months.
Several years ago, researchers found that 80 percent of patients with uveal melanoma had mutations to GNAQ or GNA11, genes that activate signals in the mitogen-activated protein kinase (MAPK) pathway. Dr. Schwartz and others subsequently demonstrated that inhibition of MEK, a key enzyme in the MAPK pathway, can inhibit the growth of uveal melanoma cells in the laboratory. Dr. Schwartz’s laboratory was the first to show this with selumetinib.
In 2013, Dr. Schwartz and his colleagues launched the first large-scale, Phase II, randomized trial of selumetinib. One hundred and one patients with metastatic uveal melanoma at 15 centers in the United States and Canada were randomized to receive either selumetinib or standard chemotherapy. Those in the chemotherapy group could receive selumetinib at any time if they showed signs of disease progression.
Median progression-free survival among patients receiving selumetinib was more than double that of patients receiving chemotherapy (15.9 weeks vs. 7 weeks). Forty-nine percent of patients treated with selumetinib exhibited tumor regression, compared with none in the chemotherapy group.
Median overall survival for patients on selumetinib was 11.8 months, compared with 9.1 months for those on chemotherapy, but the difference was not statistically significant. “We suspect that there may have been improvement in survival in the selumetinib group, but it was unclear, because patients who didn’t respond to chemotherapy were allowed to cross over to selumetinib,” said Dr. Schwartz. “That’s something we hope to clarify in a follow-up study that is now under way.”
The vast majority of patients taking selumetinib experienced side effects, including rash, swelling, and visual changes. Most of the side effects were considered manageable, although 37 percent of patients required at least one dose reduction and 6 percent discontinued therapy.
Dr. Schwartz thinks that treatment of uveal melanoma will ultimately involve rational drug design and a combination of drugs, similar to the approach used to combat HIV infection. “In preclinical studies, we’ve shown that when a MEK inhibitor was combined with an Akt inhibitor, which affects another cancer-related pathway, the results were better than when using MEK alone,” he said.
“Overall,” Dr. Schwartz added, “the study underscores the importance of rational drug design, in which drugs are designed to interact with specific molecular pathways involved in a particular disease. This is a huge improvement over chemotherapy, which is basically a blanket approach to cancer that does not directly address the underlying biology.”
About:
The paper is titled, “Effects of Selumetinib vs. Chemotherapy on Progression-Free Survival in Uveal Melanoma.” The other contributors are Richard D. Carvajal (Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY), Jeffrey A. Sosman (Vanderbilt University Medical Center, Nashville, TN), Jorgr Fernando Quevedo (Mayo Clinic, Rochester, MN), Mohammed M. Milhem (University of Iowa Hospitals and Clinic, Iowa City, IA), Anthony Michael Joshua (Princess Margaret Cancer Centre, Toronto, Canada), Ragini R. Kudchadkar (H. Lee Moffitt Cancer Center, Tampa, FL), Gerald P. Linette (Washington University, St. Louis, MO), Thomas F. Gajewksi (University of Chicago, Chicago, IL), Jose Lutzky (Mount Sinai Comprehensive Cancer Center, Miami Beach, FL), David H. Lawson (Winship Cancer Institute of Emory University, Atlanta, GA), Christopher D. Lao (University of Michigan, Ann Arbor, MI), Patrick J. Flynn (Metro Minnesota Community Clinical Oncology Program, Minneapolis-St. Paul, MN), Mark R. Albertini (University of Wisconsin, Madison, WI), Takami Sato (Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA), Karl Lewis (University of Colorado, Aurora, CO), Austin Doyle (Investigational Drug Branch, National Cancer Institute, Rockville, MD), Kristin Ancell (Vanderbilt University Medical Center), Katherine S. Panageas (MSKCC), Mark Bluth (MSKCC), Cyrus Hedvat (MSKCC), Joseph Erinjeri (MSKCC), Grazia Ambrosini (MSKCC), Brian Marr (MSKCC), David H. Abramson (MSKCC and Weill Cornell), Mark Andrew Dickson (MSKCC and Weill Cornell), Jedd D. Wolchok (MSKCC and Weill Cornell), and Paul B. Chapman (MSKCC and Weill Cornell).
The authors declare no financial or other conflicts of interests.
The study was supported by grants from the National Cancer Institute (NCT01143402), the Conquer Cancer Foundation, Cycle for Survival, and the Fund for Ophthalmic Knowledge.
Columbia University Medical Center provides international leadership in basic, preclinical, and clinical research; medical and health sciences education; and patient care. The medical center trains future leaders and includes the dedicated work of many physicians, scientists, public health professionals, dentists, and nurses at the College of Physicians and Surgeons, the Mailman School of Public Health, the College of Dental Medicine, the School of Nursing, the biomedical departments of the Graduate School of Arts and Sciences, and allied research centers and institutions. Columbia University Medical Center is home to the largest medical research enterprise in New York City and State and one of the largest faculty medical practices in the Northeast. For more information, visit cumc.columbia.edu or columbiadoctors.org.
The Herbert Irving Comprehensive Cancer Center at NewYork-Presbyterian/Columbia University Medical Center encompasses preclinical and clinical research, treatment, prevention, and education efforts in cancer. The Cancer Center was initially funded by the NCI in 1972 and became a National Cancer Institute (NCI)–designated comprehensive cancer center in 1979. The designation recognizes the Center’s collaborative environment and expertise in harnessing translational research to bridge scientific discovery to clinical delivery, with the ultimate goal of successfully introducing novel diagnostic, therapeutic and preventive approaches to cancer. For more information, visit www.hiccc.columbia.edu.
NewYork-Presbyterian Hospital/Columbia University Medical Center, located in New York City, is one of the leading academic medical centers in the world, comprising the teaching hospital NewYork-Presbyterian and its academic partner, Columbia University College of Physicians and Surgeons. NewYork-Presbyterian/Columbia provides state-of-the-art inpatient, ambulatory and preventive care in all areas of medicine, and is committed to excellence in patient care, research, education and community service. NewYork-Presbyterian Hospital also comprises NewYork-Presbyterian Hospital/Weill Cornell Medical Center, NewYork-Presbyterian/Morgan Stanley Children’s Hospital, NewYork-Presbyterian Hospital/Westchester Division, NewYork-Presbyterian/The Allen Hospital and NewYork-Presbyterian/Lower Manhattan Hospital. NewYork-Presbyterian is the #1 hospital in the New York metropolitan area.and is consistently ranked among the best academic medical institutions in the nation, according to U.S.News & World Report. For more information, visit www.nyp.org.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















