Drug combination acts against aggressive chronic lymphocytic leukemia

A two-prong approach combining ibrutinib and rituximab (Rituxin®) to treat aggressive chronic lymphocytic leukemia (CLL) produced profound responses with minor side effects in a Phase 2 clinical trial at the University of Texas MD Anderson Cancer Center.
Researchers presented the results today at the 54th annual meeting of the American Society of Hematology (ASH).
“This is a patient population with a great need for more targeted therapies,” said Jan Burger, M.D., Ph.D., associate professor in MD Anderson's Department of Leukemia. Burger was lead author of the study.
“Many CLL patients, especially those with indolent or non-aggressive disease, do well on the standard treatment of chemotherapy and antibodies,” he said. “But for a certain subset of high-risk patients, treatment often fails, and remissions, if they are achieved, are short.”
According to the National Cancer Institute's Surveillance Epidemiology and End Results database, CLL is the most common type of adult leukemia in the United States. An estimated 16,000 new cases will be diagnosed this year, and about 4,600 people will die because of the disease. Median age of diagnosis is 72, and it is more common in men than women.
Although chemotherapy combinations have improved the cure rate for CLL, side effects often are severe. A sizeable number of CLL deaths are from secondary cancers caused by treatment.
Early studies showed potential
Ibrutinib, a tyrosine kinase inhibitor that thwarts B-cell receptor signaling, is a promising new targeted therapy for mature B-cell malignancies, including certain types of myeloma and lymphoma. It has been shown to be especially effective in CLL.
Over the past two years, Phase 1/2 trials at MD Anderson and other sites showed high-risk CLL patients responded as well as low-risk patients to ibrutinib. However, the response often is lessened because of persistent lymphocytosis, an increase in leukemia cells in the blood due to release of CLL cells from the tissues (lymph glands) into the blood stream. Rituximab, a well-established antibody, was added to capture the CLL cells in the blood and thereby accelerate and improve response.
“When we looked at how well the high-risk patients were doing on ibrutinib – even though it was a small number – we saw a great opportunity to find out if combining the two drugs would have a positive impact on these patients,” Burger said.
Combination tolerated well
Forty patients with high-risk CLL were enrolled in the study earlier this year. They received:
Daily oral doses of 420 mg ibrutinib throughout treatment
Weekly infusions of rituximab (375 mg/m2) weeks one through four
Monthly rituximab infusions for the next five months
At a median follow up of four months, 38 patients remained on ibrutinib therapy without disease progression. One patient died from an unrelated infectious complication, and one patient discontinued therapy due to oral ulcers.
Preliminary results: 85 percent response rate
Of 20 patients evaluated for early response at three months, 17 achieved partial remission for an overall response rate of 85%. Three achieved partial remission with persistent lymphocytosis.
Interestingly, lymphocytosis peaked earlier and the duration was shorter than with ibrutinib alone.
Treatment was well tolerated, with 13 cases of grade 3 or grade 4 toxicities, including neutropenia, fatigue, pneumonia, insomnia and bone aches. Most side effects were unrelated and transient. Many patients reported improved overall health and quality of life after three cycles of treatment.
“Although this study has a short follow-up time, we are encouraged by the fact that the vast majority of patients are responding and are able to continue on treatment, Burger said.
Development of ibrutinib for CLL crucial
Researchers said these data, together with the previous Phase 1/2 studies, emphasize the need for rapid further development of ibrutinib for high-risk CLL patients.
Pharmacyclics, the company that is developing ibrutinib, is proceeding with a Phase 3 multi-center clinical trial, in which MD Anderson will participate. Additionally, MD Anderson researchers will conduct a follow-up study on their research in high-risk CLL patients.
Other research team members from MD Anderson's Department of Leukemia included Michael Keating, M.D.; William Wierda, M.D., Ph.D.; Julia Hoellenriegel, M.S.; Alessandra Ferrajoli, M.D.; Stefan Faderl, M.D.; Susan Lerner, M.S.; Gracy Zacharian; Hagop Kantarjian, M.D.; and Susan O'Brien, M.D. Also participating were Xuelin Huang, Ph.D., of the Department of Biostatistics at MD Anderson; and Danelle James, M.D. and Joseph Buggy, Ph.D., of Pharmacyclics, Inc, Sunnyvale, CA.
Support for the study was provided by Pharmacyclics and CLL Global Research Foundation.
About MD Anderson
The University of Texas MD Anderson Cancer Center in Houston ranks as one of the world's most respected centers focused on cancer patient care, research, education and prevention. MD Anderson is one of only 41 comprehensive cancer centers designated by the National Cancer Institute. For nine of the past 11 years, including 2012, MD Anderson has ranked No. 1 in cancer care in “America's Best Hospitals,” a survey published annually in U.S. News & World Report.
Media Contact
More Information:
http://www.mdanderson.orgAll latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles
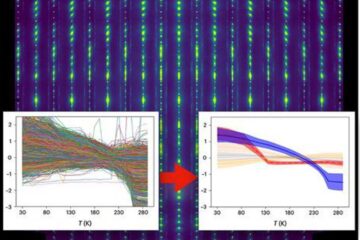
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
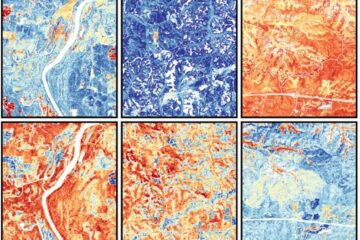
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















