A novel insight into cardiac function: Development of a new model of spontaneous oscillatory contraction
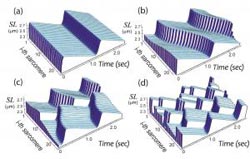
Figure 1. Patterns of SPOC identified by the series connection model. (a) Synchronized (in-phase) SPOC; (b) propagating (traveling wave) SPOC; (c) propagation-interrupted (disrupted traveling wave) SPOC; and (d) random (out-of-phase) SPOC. The letters correspond to those in Figure 2. SL: sarcomere length. The oscillation waveforms are saw-tooth shaped, irrespective of the oscillation pattern. <br>
Beginning with their discovery of spontaneous oscillatory contraction (SPOC) of muscle, Prof. Shin’ichi Ishiwata and his team at Waseda University’s Faculty of Science and Engineering have been experimentally characterizing this phenomenon over the last 25 years.
Recently, the team, in collaboration with Dr. Katsuhiko Sato of the RIKEN Center for Developmental Biology (Kobe) and Vice Director Yoshiki Kuramoto of the International Institute for Advanced Studies, has successfully developed a realistic theoretical model that provides a rational explanation for SPOC.
The model demonstrates the auto-oscillatory properties of an assembly of membraneless muscle proteins (the contractile system called myofibril), and it gives new insights into cardiac functions.
Details of this research have been published in the acclaimed international physical science journal, ‘Physical Review Letters (vol. 111, 108104; 2013)’.
————————-
PROJECT OUTLINE
————————-
Skeletal and cardiac muscles are collectively referred to as striated muscle. The contractile system (an assembly of muscle proteins) of striated muscle is a force-generating apparatus composed of many, linearly linked contractile units called sarcomeres. Sarcomeres consist of neatly aligned fibrous proteins, i.e., myosin motor proteins which hydrolyze ATP (adenosine triphosphate), and thin actin filaments. Contraction and relaxation of sarcomeres are switched on and off by calcium (Ca) concentrations fluctuating around 1 micrometer.
In cardiac muscle, a transient increase in cellular Ca concentration occurs in response to regular electrical signals produced by pacemaker cells. This leads to binding of Ca ions to the regulatory protein, troponin, which switches on thin filaments and allows thick myosin filaments to bind the thin filaments.
Subsequent conformational change in the bound myosin generates a ‘sliding force’ which causes the thick and thin filaments to slide past each other, resulting in shortening of the sarcomeres and ultimately the entire muscle fiber. Conversely, a reduction in cellular Ca concentration triggers the opposite effect; i.e., Ca ions are released from troponin, and thin filaments are switched off, leading to dissociation of myosin motors from thin actin filaments.
As a result, the contractile system relaxes and, due to an external stretching force, it regains its length. Thus, muscle contractile systems are considered to be in either one of the two, on and off states. In particular, in a muscle cell covered with membranes, the contractile system always takes either the on or off state, depending on the electrical stimuli, which is known as the ‘all-or-none law.’
It is therefore widely accepted that the contractile apparatus, responsible for force generation, is also either in the contracted (on) or relaxed (off) state (this is the textbook description).
In cardiac muscle, however, the increased cellular Ca concentration does not greatly exceed the on-off trigger level of 1 micrometer, and it always remains around this level despite the all-or-none nature of electrical signals given by pacemaker cells, although the reason behind this is unclear.
We previously established that, using skinned muscle fibers placed in a Ca buffer, each sarcomere exhibited stable auto-oscillation of contraction and elongation under the fixed Ca concentration of approximately 1 micrometer, through careful measurements of muscle fiber tension (especially using thin bundles of myofibrils ≈1 micrometer in diameter); we termed this phenomenon as spontaneous oscillatory contraction (SPOC) (see recent review by S. Ishiwata, Y. Shimamoto, and N. Fukuda; Prog. Biophys. Mol. Biol. 105, 187; 2011).
In skeletal muscle, SPOC does not readily occur even when Ca concentration is adjusted to 1 micrometer. In cardiac muscle, in contrast, SPOC quickly appears in response to a Ca concentration of ≈1 micrometer. In the case of a muscle cell composed of bundles of myofibrils, each sarcomere does oscillate (the oscillation waveform presents a ‘saw-tooth’ appearance consisting of slow shortening and quick lengthening), but the oscillation periods are not synchronized along the myofibrils and, hence, length oscillation of the entire muscle cell is rarely observed.
In 2011, we first constructed a theoretical model describing SPOC in individual sarcomeres (K. Sato, M. Ohtaki, Y. Shimamoto, and S. Ishiwata; Prog. Biophys. Mol. Biol. 105, 199; 2011). This model proposed a mechanism of SPOC behavior seen as periodical changes in sarcomere length with a saw-tooth waveform under a constant Ca concentration.
The fundamental assumptions of this model were that the balance of forces is not only parallel but also perpendicular to the long axis of sarcomeres (myofibrils), taking into account the balance of viscoelastic forces originating from the filament lattice and crossbridges (actin-bound myosin heads); the probability of crossbridge formation is dependent on the spacing of two filaments (lattice constant); and lattice constants decrease with increasing sarcomere length (i.e., volume of the lattice spacing is kept more or less constant) under the equilibrium state of relaxation. In the present study, we employed a series connection of the unit model formed elastically and next to each other, and examined whether the revised model was able to reproduce myofibril SPOC.
The results showed that the series connection model was successful in reproducing nearly all SPOC patterns we have observed to date (Figure 1). The patterns of sarcomere oscillation/contraction defined by the newer model include synchronized oscillation (in-phase SPOC); propagated oscillation along a myofibril (traveling wave SPOC); disrupted propagation of a traveling wave SPOC due to another traveling wave SPOC from elsewhere in the same myofibril colliding with or reversing the initial oscillation (disrupted traveling wave SPOC); randomly-occurring oscillation (out-of-phase SPOC); and the force generation state in the absence of oscillation (contraction) (see Figure 2 for phase diagram).
We found that these variable SPOC patterns were generated according to the number of sarcomeres in a myofibril as well as the stiffness ratio of elastic structures inside the contractile system relative to those external to the system. Our next steps are to advance the newer, one-dimensional connection model to two- and three-dimensional models in order to elucidate the true dynamics of muscle cells (bundles of myofibrils).
The successful reproduction of SPOC patterns achieved with the series connection model strongly suggests the auto-oscillatory nature of cardiac contractile system itself, meaning that muscle contractile systems are autonomous oscillators in their own right and not mere force generators (laborers) simply responding to electrical signals or Ca concentration changes.
This brings into question the role of Ca concentration fluctuations caused by electrical signals from pacemaker cells when the heart beats. We already know, however, that SPOC periods in the cardiac contractile system are correlated with the heart rate in several species of animals.
Therefore, we must explore a new regulatory mechanism in which fluctuations in Ca concentration trigger large-scale synchronization of sarcomere-specific autonomous oscillations. Depending on our future findings, we may one day need to rewrite textbooks.
Journal information
Locally and Globally Coupled Oscillators in Muscle in Physical Review Letters (vol. 111, 108104; 2013)
Authors
* Co-corresponding authors.
• Katsuhiko Sato* (katsuhiko-sato@cdb.riken.jp), Research Scientist, RIKEN Center for Developmental Biology
• Yoshiki Kuramoto, Vice Director, International Institute for Advanced Studies
• Masako Ohtaki, Invited Researcher, Faculty of Science and Engineering, Waseda University
• Yuta Shimamoto, Faculty of Science and Engineering, Waseda University (currently Postdoctoral Fellow, Rockefeller University)
• Shin’ichi Ishiwata* (Ishiwata@waseda.jp), Professor, Faculty of Science and Engineering, Waseda University; and Director, Waseda Bioscience Research Institute in Singapore (WABIOS)
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















