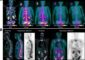
Small Assist Device Used in Emergency Case as Twin, Heart Booster Pumps for First Time in U.S.

The Bluhm Cardiovascular Institute of Northwestern Memorial Hospital recently implanted a patient with two of the smallest experimental ventricular assist devices (VADs) currently available for study in humans.
VADs are designed to assist either the right (RVAD) or left (LVAD) ventricle, or both (BiVAD) at once. This is the first time that two Heartware™ VADs have been implanted in the left and right ventricles anywhere in North America—a “game changer” in the realm of heart assist devices according to Patrick M. McCarthy, MD, chief of the hospital’s Division of Cardiac Surgery and director of the hospital’s Bluhm Cardiovascular Institute.
“The world doesn’t need the artificial heart anymore,” said Dr. McCarthy, who is also the Heller-Sacks Professor of Surgery at Northwestern University Feinberg School of Medicine. “The goal is total support of the heart. This biventricular approach achieves that without cutting out the patient’s own heart, which is what happens with artificial heart implants.”
According to Edwin C. McGee, Jr. MD, surgical director for the Bluhm Institute’s Heart Transplant and Assist Device program and the lead cardiac surgeon who performed the implant, when the patient, 44-year-old James Armstrong, was transferred to Northwestern Memorial just weeks ago, he was near death with an aggressive state of myocarditis. Myocarditis is a severe inflammation of the heart tissue that—in some rare cases—can be fatal when unaddressed.
Only about a dozen times before in Europe had the twin implant of the small Heartware™ VAD been performed, and now the approach would be Armstrong’s best chances for survival. The Heartware™ VAD is under trial in the U.S. as an LVAD. Mr. Armstrong did not qualify for the current trial and was able to have the device implanted under a process known as “emergency use”. Emergency use is defined as the use of an investigational article with a human subject in a life-threatening situation. Although such uses are not yet approved by the U.S. Food and Drug Administration, they are allowed when there is no standard acceptable treatment available and there is not sufficient time to obtain approval from an Institutional Review Board.
“As we do for all of our patients, we wanted the very best for Jim,” said McGee. “Standard BiVAD pumps sit outside of the body and are plagued by an extremely high complication and mortality rate.”
McGee added that another reason to study this system is because the configuration leaves patients’ hearts intact. This, he says, lends itself to the possibility that in some rare cases the heart may actually recover. “Naturally, that remains to be seen—but it’s possible,” he says. This probability is obliterated with artificial hearts because essential components of the natural heart are removed.
McGee adds that one of the greatest advantages to patients is that the pumps are contained (implanted) completely within the chest. There are two small controlling leads in the body that connect to a small monitor and power source outside the body that can be carried in two small shoulder pouches.
“Using this device in a biventricular support configuration may offer total heart support to more individuals with improved quality of life and hopefully fewer complications, than with the currently approved devices,” added McGee.
The American Heart Association estimates that an average of 300,000 people die every year from heart failure—and roughly 10,000 of them qualify for heart transplant. Due to lack of donor organs roughly only 2000 cardiac transplants are performed each year. Assist devices such as the ones Armstrong received are becoming an increasingly important therapy to help individuals with advanced heart failure.
Dr. McGee is a paid consultant for HeartWare™.
Media Contact
Kris Lathan, Director
Northwestern Memorial Hospital
312-926-2963
klathan@nmh.org
Media Contact
More Information:
http://www.nmh.org/nm/bivad+releaseAll latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…