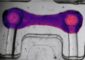
Bone-hard biomaterial

In the surgical procedure the torn ligament is replaced by a piece of tendon from the leg, which is fixed to the bone by means of an interferential screw. The problem is that the screws are made of titanium. After a certain time the patient has to undergo a further surgery so that the material can be removed.
Researchers at the Fraunhofer Institute for Manufacturing Engineering and Applied Materials Research (IFAM) in Bremen want to spare cruciate ligament victims and other bone patients this additional procedure. They have therefore developed a screw which is biocompatible and also biodegradable over time. »We have modified biomaterials in such a way that they can be formed into robust bioactive and resorbable screws by means of a special injection molding process,« explains Dr. Philipp Imgrund, head of the biomaterial technology department at IFAM.
»Depending on the composition they biodegrade in 24 months.« Biodegradable screws made of polylactic acid are already used in the medical field, but they have the disadvantage that when they degrade they can leave holes in the bone. The IFAM researchers have therefore improved the material and developed a moldable composite made of polylactic acid and hydroxylapatite, a ceramic which is the main constituent of the bone mineral. »This composite possesses a higher proportion of hydroxylapatite and promotes the growth of bone into the implant,« says Imgrund.
The engineers at IFAM have developed a granulate from the biomaterials which can be precision-processed using conventional injection molding methods, obviating the need for any post-processing such as milling. The complex geometry is achieved in a net-shape process, producing a robust screw. The properties of this prototype come very close to those of real bone. Its compressive strength is more than 130 newtons per square millimeter, whereas real bone can withstand between 130 and 180. What's more, the injection molding process has a positive side effect. Normally, the powder injection molded part has to be compressed at very high temperatures of up to 1400° Celsius. »We only need 140 degrees for our composite materials,« says Imgrund. In future the engineers intend to develop other bioimplants using their energy-saving process.
Media Contact
More Information:
http://www.ifam.fraunhofer.deAll latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…