How the worm turns

New research by scientists at the University of Massachusetts Medical School shows at the single cell level how an external stimulus sets off a molecular chain reaction in the transparent roundworm C. elegans, a process in which a single neurotransmitter coordinates and times two separate actions.
These findings shed new light on how neurons translate sensory input into actions and may one day pave the way to understanding how misfiring neurons contribute to motor symptoms in neurological diseases such as Parkinson's disease. Details of the study were published online by PLOS Biology.
“We've known the broad outline of how a behavior circuit works-a stimulus starts a neuronal cascade, which ultimately activates a muscle cell-for decades,” said Mark Alkema, PhD, assistant professor of neurobiology. “The details about how this process works, however, such as which neurotransmitters act through which receptors in which neurons have remained a mystery for even the simplest of behaviors.
“This research provides an answer to the simple question of how the worm turns around and the broader question of how a behavioral sequence is produced on a sub-cellular level. In time, understanding voluntary movement in humans will require answering the same questions about the timing and location of neurons and neurotransmitters-only in the infinitely more complex variety of circuits in the human nervous system,” said Dr. Alkema.
Roundworms move by alternately relaxing and contracting ventral and dorsal muscles along both sides of its body. As the animal moves forward, it uses its head to probe for possible threats. A gentle touch to the head of the worm initiates an escape response resulting in the animal ceasing head movements and quickly moving backwards. This initial reaction is closely followed by a deep ventral turn allowing it to move away in the opposite direction.
Earlier studies have shown that tyramine, a monoamine neurotransmitter akin to noradrenaline in humans, is involved in the C. elegans escape response. Specifically, C. elegans have a pair of tyraminergic motor neurons that are essential for coordinating the initial suppression of head movement and the backing response. These neurons release tyramine, which works through a fast-acting ion channel called LGC-55 to inhibit forward movement and relax the neck muscles. How the animal coordinates this movement with the subsequent deep turn that allows it to complete the change in direction and move away from the threat, however, was unknown. In this study, the authors provide evidence that links this initial phase of the escape response to the later stages in which the worm makes a sharp turn and navigates away from the danger.
When C. elegans are placed on a surface containing a high concentration of tyramine they become immobilized. Alkema and colleagues found that this paralysis could be overcome by mutating the C. elegans gene responsible for encoding the G-protein coupled receptor SER-2. Additionally, they found that the SER-2 receptor was active in a set of 13 neurons residing along the ventral nerve cord. The synapses of these neurons were connected to corresponding ventral muscles cells along one side of the worm's body.
Further experiments revealed that the same monoamine neurotransmitter-tyramine-responsible for the initial phase of the escape response was also responsible for activating the slow-acting G-protein coupled receptor SER-2. Activation of this receptor inhibited release of the neurotransmitter GABA and facilitated contraction of the ventral muscles, allowing the animal to complete its turn and resume movement in the opposite direction.
“This study shows how tyramine works through separate receptors to produce a complex behavior requiring the temporal coordination of independent motor programs,” said Alkema. “Acting through the fast-acting ionotropic receptor LGC-55, the animal completes the initial movement by ceasing head movement and backing away. At the same time, the slow-acting SER-2 receptor is also being activated by tyramine to complete the turn and facilitate movement in the opposite direction.
“It is the different receptors that allow for the coordination of these actions by the same neurotransmitter,” said Alkema. “This indicates that tyramine, much like adrenergic signaling in mammals, coordinates different aspects of the flight response. It's possible that temporally coordinated activation of ionotropic and metabotropic receptors may be a common signaling motif employed across organisms to orchestrate behavioral responses and is something we will be pursing further.”
About the University of Massachusetts Medical School
The University of Massachusetts Medical School, one of the fastest growing academic health centers in the country, has built a reputation as a world-class research institution, consistently producing noteworthy advances in clinical and basic research. The Medical School attracts more than $250 million in research funding annually, 80 percent of which comes from federal funding sources. The mission of the Medical School is to advance the health and well-being of the people of the commonwealth and the world through pioneering education, research, public service and health care delivery with its clinical partner, UMass Memorial Health Care. For more information, visit http://www.umassmed.edu.
Media Contact
More Information:
http://www.umassmed.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
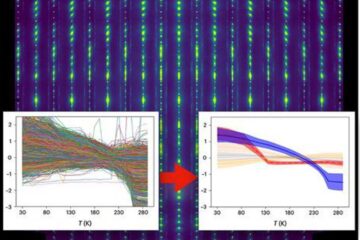
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
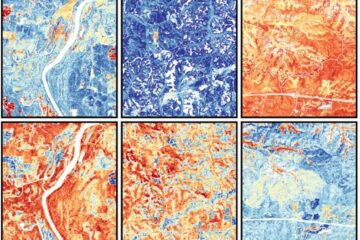
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















