Touch of gold improves nanoparticle fuel-cell reactions
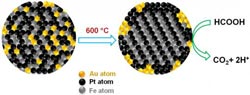
Midas touch on the nanoscale<br> Gold atoms create orderly places for iron and platinum atoms, then retreat to the periphery of the fuel cell, where they scrub carbon monoxide from fuel reactions. The tighter organization and cleaner reactions extend the cell's performance life. Credit: Sun Lab/Brown University <br>
The key is the addition of gold: It yields a more uniform crystal structure while removing carbon monoxide from the reaction. Results published in the Journal of the American Chemical Society.
Advances in fuel-cell technology have been stymied by the inadequacy of metals studied as catalysts. The drawback to platinum, other than cost, is that it absorbs carbon monoxide in reactions involving fuel cells powered by organic materials like formic acid. A more recently tested metal, palladium, breaks down over time.
Now chemists at Brown University have created a triple-headed metallic nanoparticle that they say outperforms and outlasts all others at the anode end in formic-acid fuel-cell reactions. In a paper published in the Journal of the American Chemical Society, the researchers report a 4-nanometer iron-platinum-gold nanoparticle (FePtAu), with a tetragonal crystal structure, generates higher current per unit of mass than any other nanoparticle catalyst tested. Moreover, the trimetallic nanoparticle at Brown performs nearly as well after 13 hours as it did at the start. By contrast, another nanoparticle assembly tested under identical conditions lost nearly 90 percent of its performance in just one-quarter of the time.
“We’ve developed a formic acid fuel-cell catalyst that is the best to have been created and tested so far,” said Shouheng Sun, chemistry professor at Brown and corresponding author on the paper. “It has good durability as well as good activity.”
Gold plays key roles in the reaction. First, it acts as a community organizer of sorts, leading the iron and platinum atoms into neat, uniform layers within the nanoparticle. The gold atoms then exit the stage, binding to the outer surface of the nanoparticle assembly. Gold is effective at ordering the iron and platinum atoms because the gold atoms create extra space within the nanoparticle sphere at the outset. When the gold atoms diffuse from the space upon heating, they create more room for the iron and platinum atoms to assemble themselves. Gold creates the crystallization chemists want in the nanoparticle assembly at lower temperature.
Gold also removes carbon monoxide (CO) from the reaction by catalyzing its oxidation. Carbon monoxide, other than being dangerous to breathe, binds well to iron and platinum atoms, gumming up the reaction. By essentially scrubbing it from the reaction, gold improves the performance of the iron-platinum catalyst. The team decided to try gold after reading in the literature that gold nanoparticles were effective at oxidizing carbon monoxide — so effective, in fact, that gold nanoparticles had been incorporated into the helmets of Japanese firefighters. Indeed, the Brown team’s triple-headed metallic nanoparticles worked just as well at removing CO in the oxidation of formic acid, although it is unclear specifically why.
The authors also highlight the importance of creating an ordered crystal structure for the nanoparticle catalyst. Gold helps researchers get a crystal structure called “face-centered-tetragonal,” a four-sided shape in which iron and platinum atoms essentially are forced to occupy specific positions in the structure, creating more order. By imposing atomic order, the iron and platinum layers bind more tightly in the structure, thus making the assembly more stable and durable, essential to better-performing and longer-lasting catalysts.
In experiments, the FePtAu catalyst reached 2809.9 mA/mg Pt (mass-activity, or current generated per milligram of platinum), “which is the highest among all NP (nanoparticle) catalysts ever reported,” the Brown researchers write. After 13 hours, the FePtAu nanoparticle has a mass activity of 2600mA/mg Pt, or 93 percent of its original performance value. In comparison, the scientists write, the well-received platinum-bismuth nanoparticle has a mass activity of about 1720mA/mg Pt under identical experiments, and is four times less active when measured for durability.
The researchers note that other metals may be substituted for gold in the nanoparticle catalyst to improve the catalyst’s performance and durability.
“This communication presents a new structure-control strategy to tune and optimize nanoparticle catalysis for fuel oxidations,” the researchers write.
Sen Zhang, a third-year graduate student in Sun’s lab, helped with the nanoparticle design and synthesis. Shaojun Guo, a postdoctoral fellow in Sun’s lab performed electrochemical oxidation experiments. Huiyuan Zhu, a second-year graduate student in Sun’s lab, synthesized the FePt nanoparticles and ran control experiments. The other contributing author is Dong Su from the Center for Functional Nanomaterials at Brookhaven National Laboratory, who analyzed the structure of the nanoparticle catalyst using the advanced electron microscopy facilities there.
The U.S. Department of Energy and the Exxon Mobil Corporation funded the research.
Editors: Brown University has a fiber link television studio available for domestic and international live and taped interviews, and maintains an ISDN line for radio interviews. For more information, call (401) 863-2476.
Media Contact
More Information:
http://www.brown.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















