Sorting Water Molecules
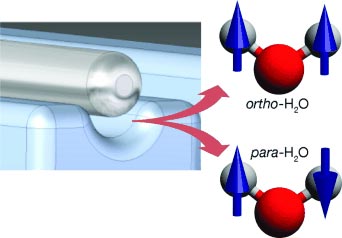
Not all water is equal—at least not at the molecular level. There are two versions of the water molecule, para and ortho water, in which the spin states of the hydrogen nuclei are different. In the journal Angewandte Chemie, German researchers have now reported the successful separation of the two forms.
Spin is a quantum mechanical value that can be visualized as the intrinsic angular momentum of a particle rotating around its own axis. A hydrogen nucleus (proton) can adopt two different states, comparable to rotation clockwise and counterclockwise.
In the case of water, the nuclear spins of the two—indistinguishable—protons can be combined in four different ways: one antisymmetric and three symmetric wavefunctions. Water adopting the antisymmetric wavefunction is called para water, whereas water adopting one of the symmetric ones is called ortho water. Because switching from one state to the other is “forbidden” due to quantum-mechanical symmetry rules, the two spin isomers cannot interconvert without external influences such as collisions.
A team at the Center for Free Electron Laser Science (DESY and University of Hamburg) led by Jochen Küpper and Daniel Horke has now successfully separated the two spin isomers and prepared isolated, highly pure samples of para and ortho water.
The challenge was to “freeze” the spin states by diluting single water molecules to such an extent that they could no longer collide with each other. To achieve this, the researchers use a noble gas high-pressure chamber charged with one droplet of water. When the chamber’s pulse valve is opened, the mixture shoots into a vacuum chamber at supersonic speeds, cooling rapidly due to the extremely fast expansion. This results in a focused beam of very cold water molecules that are so far from each other that they cannot induce a spin flip in each other.
For the separation, the researchers made use of the fact that para and ortho water molecules do not have the same quantum states. In a strong inhomogeneous electric field, the accelerated water molecules are deflected from their flight path by a different amount depending on their quantum state.
Possible applications for spin-pure water extend to many fields. Astrophysicists have determined that the ratio of ortho water to para water in interstellar ice is different from what is expected. Spin-pure water could enable revealing laboratory experiments. Techniques such as nuclear magnetic resonance spectroscopy (NMR) could benefit as their sensitivity could be increased by the use of para water in the hydration shells of proteins, improving the determination of their structure.
About the Author
Dr. Jochen Küpper is a team leader at DESY and Professor of Experimental Physics at the University of Hamburg. His research field comprises molecular physics and physical chemistry, focusing on the interconnection of structure, dynamics, and function of molecules. He already received several awards, including the Nernst-Haber-Bodenstein Award of the German Bunsen Society in 2009 and a Consolidator Grant „COMOTION“ of the European Research Council in 2013.
Author: Jochen Küpper, DESY, Hamburg (Germany), http://desy.cfel.de/cid/cmi/
Title: Separating Para and Ortho Water
Angewandte Chemie International Edition
Permalink to the original article: http://dx.doi.org/10.1002/anie.201405986
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















