Showing the way to improved water-splitting catalysts
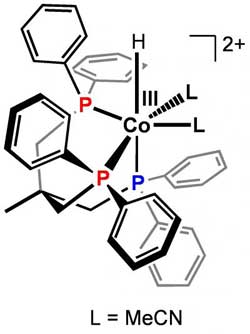
Harry Gray's group at Caltech added a set of ligands to cobalt, slowing the reaction so that they could observe a key intermediate and then determine the chemical mechanism.<br><br>Credit: Caltech/Marinescu et al.<br>
Scientists and engineers around the world are working to find a way to power the planet using solar-powered fuel cells.
Such green systems would split water during daylight hours, generating hydrogen (H2) that could then be stored and used later to produce water and electricity. But robust catalysts are needed to drive the water-splitting reaction.
Platinum catalysts are quite good at this, but platinum is too rare and expensive to scale up for use worldwide. Several cobalt and nickel catalysts have been suggested as cheaper alternatives, but there is still plenty of room for improvement. And no one has been able to determine definitively the mechanism by which the cobalt catalysts work, making it difficult to methodically design and construct improved catalysts.
Now chemists at the California Institute of Technology (Caltech) have determined the dominant mechanism for these cobalt catalysts. Their findings illuminate the road to the development of better catalysts—even suggesting a route to the development of catalysts based on iron, an element that is plentiful and cheap and could offer part of the answer to our energy woes.
“We've worked out this mechanism, and now we know what to do to make a really great catalyst out of something that's really cheap as dirt,” says Harry Gray, the Arnold O. Beckman Professor of Chemistry at Caltech and senior author of a paper that describes the findings in the current issue of the Proceedings of the National Academy of Sciences (PNAS). “This work has completely changed our thinking about which catalyst designs to pursue.”
A major barrier to improving the performance of man-made catalysts has been the lack of understanding of the mechanism—the chemical pathway that such catalysts follow leading to the production of hydrogen. As with any multistep manufacturing project, chemists need to know what is involved in each reaction that takes place—what goes in, what changes take place, and what comes out—in order to maximize efficiency and yield.
Three mechanisms have been suggested for how the cobalt catalysts help make hydrogen—one proposed by a French team, one developed by Caltech researchers, including Nate Lewis and Jonas Peters, and a third suggested more recently by a former graduate student in Gray's group, Jillian Dempsey (PhD '10). Until now, no one has managed to prove definitively which mechanisms actually occur or whether one was dominant, because the reactions proceed so quickly that it is difficult to identify the chemical intermediates that provide evidence of the reactions taking place.
These cobalt catalysts are complexes that involve the metal bound to many different functional groups, or ligands. In the current study, Caltech postdoctoral scholar Smaranda Marinescu was able to add a new set of ligands to cobalt, making the reaction slow down to the point where the researchers could actually observe the key intermediate using nuclear magnetic resonance (NMR) spectroscopy. “Once we could see that key intermediate by NMR and other methods, we were able to look at how it reacted in real time,” Gray says. They saw that Dempsey's mechanism is the predominant pathway that these catalysts use to generate hydrogen. It involves a key reactive intermediate gaining an extra electron, forming a compound called cobalt(II)-hydride, which turns out to be the mechanism's active species.
In a previous PNAS paper, work by Gray and lead author Carolyn Valdez suggested that the Dempsey mechanism was the most likely explanation for the detected levels of activity. The new paper confirms that suggestion.
“We now know that you have to put another electron into cobalt catalysts in order to get hydrogen evolution,” Gray says. “Now we have to start looking at designs with ligands that can accept that extra electron or those that can make atomic cobalt, which already has the extra electron.”
Gray's group is now working on this latter approach. Moreover, these results give his group the information they need to develop an extremely active iron catalyst, and that will be their next big focus.
“We know now how to make a great catalyst,” he says. “That's the bottom line.”
In addition to Marinescu and Gray, Jay Winkler, a faculty associate and lecturer at Caltech, was also a coauthor on the paper, “Molecular mechanisms of cobalt-catalyzed hydrogen evolution.” The work was supported by the National Science Foundation Center for Chemical Innovation in Solar Fuels as well as Chevron Phillips Chemical.
Written by Kimm Fesenmaier
Media Contact
More Information:
http://www.caltech.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















