Research may explain how foremost anticancer 'guardian' protein learned to switch sides
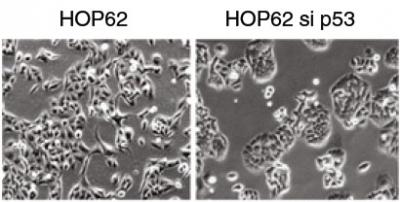
The protein p53-psi, a variant of the well-known anti-cancer guardian protein p53, can reprogram cells to "switch sides" and acquire pro-metastatic features. Pro-metastatic cells naturally expressing p53-psi are seen in LEFT panel. On RIGHT, when p53-psi is silenced, these cells reacquire the characteristics of non-metastatic cells. Credit: Sordella lab, Cold Spring Harbor Laboratory
Called p53, this protein has been called “the guardian of the genome.” It normally comes to the fore when healthy cells sense damage to their DNA caused by stress, such as exposure to toxic chemicals or intense exposure to the sun's UV rays.
If the damage is severe, p53 can cause a cell to commit preprogrammed cell-death, or apoptosis. Mutant versions of p53 that no longer perform this vital function, on the other hand, are enablers of many different cancers.
Cancer researcher Dr. Raffaella Sordella, a CSHL Associate Professor, and colleagues, today report in Proceedings of the National Academy of Sciences the discovery of a p53 cousin they call p53-psi (the Greek letter “psi”). It is a previously unknown variant of the p53 protein, generated by the same gene, called TP53 in humans, that gives rise to other forms of p53.
Sordella and colleagues observed that p53-psi, when expressed, reduces the expression of a molecular glue called E-cadherin, which normally keeps cells in contact within epithelial tissue, the tissue that forms the lining of the lung and many other body organs.
This is accompanied by expression of key cellular markers associated with tumor invasiveness and metastatic potential. (These are markers of EMT, or epithelial-to-mesenchymal transition.) Consistently, Sordella and her team found levels of p53-psi to be elevated in early-stage lung tumors with poor prognosis.
Careful investigation revealed that p53-psi generates pro-growth effects by interacting with a protein called cyclophillin D (CypD), at the membrane of the cell's energy factories, the mitochondria, and by spurring the generation of oxidizing molecules called reactive oxygen species (ROS).
p53-psi was found by the team to be inherently expressed in tumors but also in injured tissue. “This is intriguing,” Sordella says, “because generation of cells bearing characteristics of those seen in wound healing has been seen previously, in tumors.”
It is possible, Sordella says, that more familiar p53 mutants associated with tumor growth and metastasis may have “hijacked” those abilities from the program used by p53-psi; to promote healing during tissue injury. A cellular program, in other words, that evolved over eons to heal may have been hijacked by mutant p53 to enable cancers to spread out of control.
The team is currently investigating p53-psi in wound healing to help clarify its role. Confirmation would lend support to the theory that mutant p53 hijacks that function to help advance pro-metastatic processes in cancer.
The research discussed in this release was funded by a grant from the Damon Runyon Cancer Research Foundation.
“p53-psi is a transcriptionally inactive p53 isoform able to reprogram cells toward a metastatic-like state” appears online ahead of print the week of July 28, 2014 in Proceedings of the National Academy of Sciences. The authors are: Serif Senturk, Zhan Yao, Matthew Camiolo, Brendon Stiles, Trushar Rathod, Alice M. Walsh, Alice Nemajerova, Matthew J. Lazzara, Nasser K. Altorki, Adrian Krainer, Ute M. Moll, Scott W. Lowe, Luca Cartegni and Raffaella Sordella. The paper can be obtained at: http://www.pnas.org/content/early/recent
About Cold Spring Harbor Laboratory
Founded in 1890, Cold Spring Harbor Laboratory (CSHL) has shaped contemporary biomedical research and education with programs in cancer, neuroscience, plant biology and quantitative biology. CSHL is ranked number one in the world by Thomson Reuters for the impact of its research in molecular biology and genetics. The Laboratory has been home to eight Nobel Prize winners. Today, CSHL's multidisciplinary scientific community is more than 600 researchers and technicians strong and its Meetings & Courses program hosts more than 12,000 scientists from around the world each year to its Long Island campus and its China center. For more information, visit http://www.cshl.edu.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















