Newly discovered protein kills anthrax bacteria by exploding their cell walls
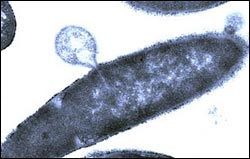
A bacterium’s final gasp. A bacillus bacterium, a close relative of anthrax, begins to explode after being treated with PlyPH. The PlyPH protein, discovered by Rockefeller scientists, offers several advantages over existing anthrax treatments.
“Anthrax is the most efficient biowarfare agent. Its spores are stable and easy to produce, and once someone inhales them, there is only a 48-hour window when antibiotics can be used,” says Fischetti. “We’ve found a new protein that could both potentially expand that treatment window and be used as a large-scale decontaminant of anthrax spores.” Because anthrax spores are resistant to most of the chemicals that emergency workers rely on to sterilize contaminated areas, a solution based on the protein would be a powerful tool for cleaning up after an anthrax attack.
All bacteria, anthrax included, have natural predators called bacteriophage. Just as viruses infect people, bacteriophage infect bacteria, reproduce, and then kill their host cell by bursting out to find their next target. The bacteriophage use special proteins, called lysins, to bore holes in the bacteria, causing them to literally explode. Fischetti and colleagues identified one of these lysins, called PlyG, in 2004, and showed that it could be used to help treat animals and humans infected by anthrax. Now, they have identified a second lysin, which they have named PlyPH, with special properties that make it not only a good therapeutic agent, but also useful for large-scale decontamination of areas like buildings and military equipment.
The new protein has several advantages. Most lysins, including PlyG, are only active in a very specific pH range of six to seven, so that they work very effectively in our bloodstream, but may not useful in many environmental conditions. “PlyPH works in an extremely wide pH range, from as low as four to as high as eight,” says Fischetti. “I don’t know of any other lytic enzyme that has such a broad range of activity.”
In addition, PlyPH, like PlyG, is highly specific in terms of the types of bacteria it affects. When Fischetti and colleagues added PlyPH to different bacterial species, only the anthrax bacteria were killed. This is a great benefit over antibiotics, which kill many different kinds of bacteria, including many helpful species. Because it is so specific, the chances of anthrax becoming resistant to PlyPH, as it is to many of the antibiotics currently available to treat it, are extremely low.
“We have never seen bacterial resistance to a lysin,” says Fischetti. “PlyPH and PlyG are probably the most specific lysins we, or anyone, has ever identified — they only kill anthrax and its very close relatives. This feature, and the wide pH range offered by PlyPH, is why we think it could be used as an environmental decontaminant.”
Fischetti hopes to combine PlyPH with a non-toxic aqueous substance developed by a group in California that will germinate any anthrax spores it comes in contact with. As the spores germinate, the PlyPH protein will kill them, usually in a matter of minutes. The combined solution could be used in buildings, on transportation equipment, on clothing, even on skin, providing a safe, easy way to fight the spread of anthrax in the event of a mass release.
Journal of Bacteriology 188(7): 2711-2714 (April 2006)
Media Contact
More Information:
http://www.rockefeller.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















