UCR Researchers Grow Bone Cells on Carbon Nanotubes
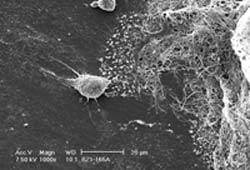
Bone cells appear as a clump at left, carbon nanotubes appear on the right.
A paper published in Nano Letters is first to show that bone cells will adhere to and grow on a carbon nanotube scaffold.
Researchers at the University of California, Riverside have published findings that show, for the first time, that bone cells can grow and proliferate on a scaffold of carbon nanotubes.
The paper, titled Bone Cell Proliferation on Carbon Nanotubes, appears in the March 8 edition of Nano Letters, a journal of the American Chemical Society. Lead author, Laura Zanello, is an assistant professor of biochemistry at UCR and was joined by UCR colleagues, graduate students Bin Zhao and Hui Hu, and Robert C. Haddon, distinguished professor of chemistry and of chemical and environmental engineering.
Zanello’s paper builds on previous research by Haddon which showed that carbon nanotubes could be chemically compatible with bone cells.
Zanello’s experiment put Haddon’s findings to the test and found that the nanotubes, 100,000 times finer than a human hair, are an excellent scaffold for bone cells to grow on.
“In the past scientists have been plagued by toxicity issues when combining carbon nanotubes with living cells,” Zanello said. “So we have been looking for the most pure nanotubes we could get to reduce the presence of heavy metals that are frequently introduced in the manufacturing process.”
She credited Haddon’s graduate student Zhao, now a postgraduate researcher at the Oak Ridge National Laboratory, with manufacturing highly pure nanotubes for her to work with.
Some of the carbon nanotubes were chemically treated and others were not, then they were combined with rat bone cells to determine which combination or combinations worked best. Non-treated and electrically-neutral nanotubes emerged as the best scaffolds for bone growth.
Because carbon nanotubes are not biodegradable, they behave like an inert matrix on which cells can proliferate and deposit new living material, which becomes functional, normal bone, according to the paper. They therefore hold promise in the treatment of bone defects in humans associated with the removal of tumors, trauma, and abnormal bone development and in dental implants, Zanello added.
More research is needed to determine how the body will interact with carbon nanotubes, specifically in its immune response, the paper states.
“We hope to look at the atomic interactions between living matter and synthetic scaffolds so we can come up with material that can interact at the nanolevel with living cells,” Zanello said.
The University of California, Riverside is a major research institution. Key areas of research include nanotechnology, genomics, environmental studies, digital arts and sustainable growth and development. With a current undergraduate and graduate enrollment of more than 16,600, the campus is projected to grow to 21,000 students by 2010. Located in the heart of Inland Southern California, the nearly 1,200-acre, park-like campus is at the center of the region’s economic development. Visit www.ucr.edu or call 951-UCR-NEWS for more information.
Media Contact
More Information:
http://www.ucr.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















