Sisyphean movement of motor proteins may help preserve DNA integrity
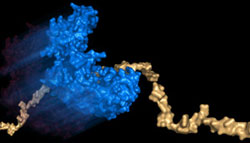
A helicase (blue) moves rapidly on a highly flexible DNA track. Such movement may prevent the accumulation of toxic proteins on the DNA. Graphic courtesy Taekjip Ha
Researchers studying how proteins called helicases travel along strands of DNA have found that when the proteins hit an obstacle they snap back to where they began, repeating the process over and over, possibly playing a preventative role in keeping the genome intact.
Taekjip Ha, a professor of physics at the University of Illinois at Urbana-Champaign and a Howard Hughes Medical Institute investigator, likens the biological scenario to Boston Red Sox baseball; the team rolls along only to hit a late-season obstacle called the New York Yankees. Then, like the always-anticipated annual cry from Chicago Cubs fan, it’s back to square one next year.
However, instead of causing more misery, as is the case for a baseball fan, this motor protein’s starting over may serve a beneficial purpose, clearing other, undesired proteins from the DNA, Ha said. The research was done in vitro, using purified proteins and studied with a technique that visualizes individual molecules on DNA. Whether the scenario plays out in real cells in not known and under exploration.
The discovery appears in the Oct. 27 issue of the journal Nature, along with a separate “News & Views” article by Eckhard Jankowsky, a biochemist at the Center for RNA Molecular Biology in Case Western University’s School of Medicine, who wrote about the potential importance of the findings.
Ha’s postdoctoral fellow Sua Myong led the study, looking at the Rep helicase from an E-coli bacterium. Rep is known to be involved in restarting DNA replication stalled by DNA damage. As a single protein, a monomer, Rep can travel one way along a single strand of DNA but by itself cannot unzip it. Rep’s progress was visualized using the single molecule fluorescence resonance energy transfer (FRET) technique that Ha had developed.
By tagging the protein and DNA with green and red dyes, Myong measured FRET changes as Rep traveled along single DNA strands, which are short segments extending out from double strands. Each time the protein reached either the junction of the full double-stranded DNA or hit an artificially created protein obstacle, Rep instantly returned to near the beginning of the single strand on which it had initially bound.
Upon closer examination using FRET, researchers discovered that Rep’s configuration gradually closed as it reached the obstacle in its path. Then, conformational changes of Rep allow it to grab and transfer to the end of the single-stranded DNA, leading to the next cycle.
“Although the very flexible single strand of DNA likely bombards the protein constantly, the protein doesn’t seem to pay attention to this overture until it hits a physical blockade,” Ha said.
Researchers had theorized that obstacles would force motor proteins to disengage from DNA. “The finding was totally unexpected and may indicate a new function for the protein,” Ha said. Jankowsky wrote that scientists “should not immediately search for the helix that the enzyme unzips, but instead remember how Rep snaps back.”
In cells, single strands of DNA often occur when something is wrong, Ha said. The recycling action, he said, may represent a desirable function of the protein by keeping it engaged on a single strand, allowing time for repairs that allow normal DNA replication.
The human body has more than 200 types of helicases involved in replication, transcription, repair and other genetic processes, Ha said. Defective helicases have been linked to increased cancer risks and premature aging.
Co-authors with Myong and Ha were Ivan Rasnik, a former postdoctoral fellow who now is a professor of physics at Emory University in Atlanta; Chirlmin Joo, a doctoral student in Ha’s lab; and Timothy M. Lohman, a professor of biochemistry and molecular biophysics at the Washington University School of Medicine in St. Louis.
Media Contact
More Information:
http://www.uiuc.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…

Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















